Strong CD4+ T-Cell Responses to Ancestral and Variant Spike Proteins Are Established by NVX-CoV2373 Severe Acute Respiratory Syndrome Coronavirus 2 Primary Vaccination
- PMID: 37210741
- PMCID: PMC10503953
- DOI: 10.1093/infdis/jiad163
Strong CD4+ T-Cell Responses to Ancestral and Variant Spike Proteins Are Established by NVX-CoV2373 Severe Acute Respiratory Syndrome Coronavirus 2 Primary Vaccination
Abstract
Background: NVX-CoV2373 is an efficacious coronavirus disease 2019 (COVID-19) vaccine comprising full-length recombinant severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (rS) glycoprotein and Matrix-M adjuvant. Phase 2 of a randomized, placebo-controlled, phase 1/2 trial in healthy adults (18-84 years of age) previously reported good safety/tolerability and robust humoral immunogenicity.
Methods: Participants were randomized to placebo or 1 or 2 doses of 5-µg or 25-µg rS with 50 µg Matrix-M adjuvant 21 days apart. CD4+ T-cell responses to SARS-CoV-2 intact S or pooled peptide stimulation (with ancestral or variant S sequences) were measured via enzyme-linked immunosorbent spot assay and intracellular cytokine staining.
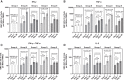
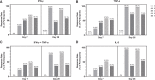
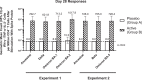
Results: A clearly discernable spike antigen-specific CD4+ T-cell response was induced after 1 dose, but markedly enhanced after 2 doses. Counts and fold increases in cells producing Th1 cytokines exceeded those secreting Th2 cytokines, although both phenotypes were clearly present. Interferon-γ responses to rS were detected in 93.5% of 2-dose 5-µg recipients. A polyfunctional CD4+ T-cell response was cross-reactive and of equivalent magnitude to all tested variants, including Omicron BA.1/BA.5.
Conclusions: NVX-CoV2373 elicits a moderately Th1-biased CD4+ T-cell response that is cross-reactive with ancestral and variant S proteins after 2 doses.
Clinical trials registration: NCT04368988.
Keywords: CD4+ T-cell response; COVID-19 vaccine; Matrix-M adjuvant; polyfunctional; variant cross-reactivity.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. L. F., R. R. M., H. Z., J. S. P., R. K., N. P., G. A., M. R., I. C., G. C., F. D., and G. M. G. are all salaried employees or contractors of Novavax. N. F. was a contractor for Novavax at the time this study was done, and is currently employed at Formative Health Pty Ltd, Casuarina NSW 2487, Australia. I. M. is an employee of Cellular Technology Ltd. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

