Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics
- PMID: 37210742
- PMCID: PMC10424881
- DOI: 10.1093/eurheartj/ehad341
Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics
Abstract
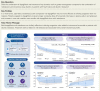
Background and aims: To examine the decongestive effect of the sodium-glucose cotransporter 2 inhibitor dapagliflozin compared to the thiazide-like diuretic metolazone in patients hospitalized for heart failure and resistant to treatment with intravenous furosemide.
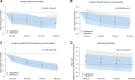
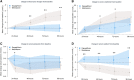
Methods and results: A multi-centre, open-label, randomized, and active-comparator trial. Patients were randomized to dapagliflozin 10 mg once daily or metolazone 5-10 mg once daily for a 3-day treatment period, with follow-up for primary and secondary endpoints until day 5 (96 h). The primary endpoint was a diuretic effect, assessed by change in weight (kg). Secondary endpoints included a change in pulmonary congestion (lung ultrasound), loop diuretic efficiency (weight change per 40 mg of furosemide), and a volume assessment score. 61 patients were randomized. The mean (±standard deviation) cumulative dose of furosemide at 96 h was 977 (±492) mg in the dapagliflozin group and 704 (±428) mg in patients assigned to metolazone. The mean (±standard deviation) decrease in weight at 96 h was 3.0 (2.5) kg with dapagliflozin compared to 3.6 (2.0) kg with metolazone [mean difference 0.65, 95% confidence interval (CI) -0.12,1.41 kg; P = 0.11]. Loop diuretic efficiency was less with dapagliflozin than with metolazone [mean 0.15 (0.12) vs. 0.25 (0.19); difference -0.08, 95% CI -0.17,0.01 kg; P = 0.10]. Changes in pulmonary congestion and volume assessment score were similar between treatments. Decreases in plasma sodium and potassium and increases in urea and creatinine were smaller with dapagliflozin than with metolazone. Serious adverse events were similar between treatments.
Conclusion: In patients with heart failure and loop diuretic resistance, dapagliflozin was not more effective at relieving congestion than metolazone. Patients assigned to dapagliflozin received a larger cumulative dose of furosemide but experienced less biochemical upset than those assigned to metolazone.
Trial registration: ClinicalTrials.gov Identifier: NCT04860011.
Keywords: Dapagliflozin; Diuretic resistance; Heart failure; Metolazone; Sodium-glucose cotransporter 2 inhibitor; Thiazide.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: S.E.Y. reports no conflicts of interest. J.O. reports no conflicts of interest. M.C.P. reports research grants from Boehringer Ingelheim, Roche, SQ Innovations, AstraZeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific, Pharmacosmos. M.C.P. reports consulting fees from Boehringer Ingelheim, Novartis, AstraZeneca, Novo Nordisk, Abbvie, Bayer, Takeda, Corvia, Cardiorentis, Pharmacosmos, Siemens, Vifor. M.C.P. reports honoraria from Boehringer Ingelheim, Novartis, Astra Zeneca, Novo Nordisk, Abbvie, Bayer, Takeda, Corvia, Cardiorentis, Pharmacosmos, Siemens, Vifor. M.C.P. is a director of Global Clinical Trial Partners Ltd. K.J.M.B. reports no conflict of interest. A.L.C. reports speaking honoraria from AstraZeneca, and consultancy honoraria from Vifor. K.F.D. reports that his employer, the University of Glasgow, has been remunerated by AstraZeneca for work related to clinical trials. K.F.D. has received speakers’ honoraria from AstraZeneca and Radcliffe Cardiology, has served on an advisory board for Us2.ai and Bayer AG, served on a clinical endpoint committee for Bayer AG, and has received grant support from Boehringer Ingelheim, Novartis and AstraZeneca (paid to his institution). P.W.X.F. reports research grant funding from Medtronic, honoraria from Pharmacosmos, and consulting fees for Medtronic. K.G. has previously received honoraria from AstraZeneca, Servier Laboratories, Boehringer Ingleheim, Novartis. An unrestricted educational grant from Biotronik limited and travel assistance from Medtronic, Boston Scientific and Abbott. C.A.H. reports no conflicts of interest. P.S.J. reports speakers’ fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals, Intas Pharma; advisory board fees from AstraZeneca, Boehringer Ingelheim, Novartis; research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc, Roche Diagnostics. P.S.J.’s employer the University of Glasgow has been remunerated for clincal trial work from AstraZeneca, Bayer AG, Novartis and NovoNordisk. Director, Global Clinical Trial Partners (GCTP). P.R.K. reports research grants from British Heart Foundation and Pharmacosmos; consulting fees from Ackea, Amgen, Boehringer Ingelheim, Pharmacosmos, Servier, and Vifor Pharma; payment for lectures from AstraZeneca, Bayer, Novartis, Pfizer, Pharmacosmos, and Vifor Pharma; support for attending meetings from Pharmacosmos; is a data safety monitoring board member for the STOP-ACE trial and EMPRESS-MI trial; and has served as Chair of the British Society for Heart Failure. G.M. reports no conflict of interest. N.N.L. reports research grants from AstraZeneca, Boehringer Ingelheim, British Heart Foundation, and Roche Diagnostics; consulting fees from AstraZeneca, Akero Therapeutics and Bristol-Myers Squibb; payment for lectures from Novartis and Roche. M.M.Y.L.'s employer, the University of Glasgow, receives grant support from AstraZeneca and Boehringer Ingelheim. He serves on clinical endpoint committees for Bayer, and steering committees for Cytokinetics. A.M. reports no conflict of interest. J.J.M. reports being an employee of AstraZeneca. E.P.’s employer has received support from Novartis for consulting work, and she has consulted for scPharmaceuticals outside of the submitted work. She has received research support from the NIH and the American Heart Association. P.S. reports being an employee of AstraZeneca. A.S. reports no conflict of interest. B.S. reports no conflict of interest. R.A.P.W. reports no conflict of interest. P.W. reports grant income from Roche Diagnostics, Astrazeneca, Boehringer Ingelheim, and Novartis, and speaker fees from Novo Nordisk and Raisio, outside the submitted work. J.J.V.M. reports payments through Glasgow University from work on clinical trials, consulting and other activities from: Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GSK, KBP Biosciences, and Novartis. Personal consultancy fees from: Alnylam Pharma., Bayer, BMS, George Clinical PTY Ltd., Ionis Pharma, Novartis, Regeneron Pharma., River 2 Renal Corporation. J.J.V.M. has received personal lecture fees from: Abbott, Alkem Metabolics, Astra Zeneca, Blue Ocean Scientific Solutions Ltd., Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharma Ltd., Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica health, Intas Pharma, J.B. Chemicals & Pharma Ltd., Lupin Pharma, Medscape/Heart.Org, ProAdWise. J.J.V.M. has received honoraria for communications, Radcliffe Cardiology, Sun Pharma, The Corpus, Translation Research Group, Translational Medicine Academy. J.J.V.M. is a director of Global Clinical Trial Partners Ltd. R.T.C. has received consultancy honoraria from Bayer and speaking honoraria from AstraZeneca.
Figures

Comment in
-
Prevention and treatment of diuretic resistance in acute heart failure: when to use which combination of diuretics?Eur Heart J. 2023 Aug 14;44(31):2978-2981. doi: 10.1093/eurheartj/ehad463. Eur Heart J. 2023. PMID: 37572039 No abstract available.

