Airway-Occluding Mucus Plugs and Mortality in Patients With Chronic Obstructive Pulmonary Disease
- PMID: 37210745
- PMCID: PMC10201404
- DOI: 10.1001/jama.2023.2065
Airway-Occluding Mucus Plugs and Mortality in Patients With Chronic Obstructive Pulmonary Disease
Abstract
Importance: Airway mucus plugs are common in patients with chronic obstructive pulmonary disease (COPD); however, the association of airway mucus plugging and mortality in patients with COPD is unknown.
Objective: To determine whether airway mucus plugs identified on chest computed tomography (CT) were associated with increased all-cause mortality.
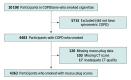
Design, setting, and participants: Observational retrospective analysis of prospectively collected data of patients with a diagnosis of COPD in the Genetic Epidemiology of COPD cohort. Participants were non-Hispanic Black or White individuals, aged 45 to 80 years, who smoked at least 10 pack-years. Participants were enrolled at 21 centers across the US between November 2007 and April 2011 and were followed up through August 31, 2022.
Exposures: Mucus plugs that completely occluded airways on chest CT scans, identified in medium- to large-sized airways (ie, approximately 2- to 10-mm lumen diameter) and categorized as affecting 0, 1 to 2, or 3 or more lung segments.
Main outcomes and measures: The primary outcome was all-cause mortality, assessed with proportional hazard regression analysis. Models were adjusted for age, sex, race and ethnicity, body mass index, pack-years smoked, current smoking status, forced expiratory volume in the first second of expiration, and CT measures of emphysema and airway disease.
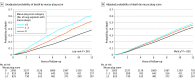
Results: Among the 4483 participants with COPD, 4363 were included in the primary analysis (median age, 63 years [IQR, 57-70 years]; 44% were women). A total of 2585 (59.3%), 953 (21.8%), and 825 (18.9%) participants had mucus plugs in 0, 1 to 2, and 3 or more lung segments, respectively. During a median 9.5-year follow-up, 1769 participants (40.6%) died. The mortality rates were 34.0% (95% CI, 32.2%-35.8%), 46.7% (95% CI, 43.5%-49.9%), and 54.1% (95% CI, 50.7%-57.4%) in participants who had mucus plugs in 0, 1 to 2, and 3 or more lung segments, respectively. The presence of mucus plugs in 1 to 2 vs 0 and 3 or more vs 0 lung segments was associated with an adjusted hazard ratio of death of 1.15 (95% CI, 1.02-1.29) and 1.24 (95% CI, 1.10-1.41), respectively.
Conclusions and relevance: In participants with COPD, the presence of mucus plugs that obstructed medium- to large-sized airways was associated with higher all-cause mortality compared with patients without mucus plugging on chest CT scans.
Conflict of interest statement
Figures

Comment in
-
Mucus Plugs and Mortality in Patients With COPD.JAMA. 2023 Oct 3;330(13):1286-1287. doi: 10.1001/jama.2023.14832. JAMA. 2023. PMID: 37787801 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

