Synaptic and circuit mechanisms prevent detrimentally precise correlation in the developing mammalian visual system
- PMID: 37211984
- PMCID: PMC10202458
- DOI: 10.7554/eLife.84333
Synaptic and circuit mechanisms prevent detrimentally precise correlation in the developing mammalian visual system
Abstract
The developing visual thalamus and cortex extract positional information encoded in the correlated activity of retinal ganglion cells by synaptic plasticity, allowing for the refinement of connectivity. Here, we use a biophysical model of the visual thalamus during the initial visual circuit refinement period to explore the role of synaptic and circuit properties in the regulation of such neural correlations. We find that the NMDA receptor dominance, combined with weak recurrent excitation and inhibition characteristic of this age, prevents the emergence of spike-correlations between thalamocortical neurons on the millisecond timescale. Such precise correlations, which would emerge due to the broad, unrefined connections from the retina to the thalamus, reduce the spatial information contained by thalamic spikes, and therefore we term them 'parasitic' correlations. Our results suggest that developing synapses and circuits evolved mechanisms to compensate for such detrimental parasitic correlations arising from the unrefined and immature circuit.
Keywords: NMDA receptor; computational model; mouse; neuroscience; precise correlation; retinal waves; thalamus; visual development.
© 2023, Tikidji-Hamburyan et al.
Conflict of interest statement
RT, GG, WG, MC No competing interests declared
Figures





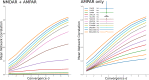





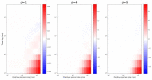

Update of
- doi: 10.1101/2022.11.11.516093
Similar articles
-
Synaptic Mechanisms Generating Orientation Selectivity in the ON Pathway of the Rabbit Retina.J Neurosci. 2016 Mar 16;36(11):3336-49. doi: 10.1523/JNEUROSCI.1432-15.2016. J Neurosci. 2016. PMID: 26985041 Free PMC article.
-
Circuitry Underlying Experience-Dependent Plasticity in the Mouse Visual System.Neuron. 2020 Apr 8;106(1):21-36. doi: 10.1016/j.neuron.2020.01.031. Neuron. 2020. PMID: 32272065 Free PMC article. Review.
-
Refinement of Spatial Receptive Fields in the Developing Mouse Lateral Geniculate Nucleus Is Coordinated with Excitatory and Inhibitory Remodeling.J Neurosci. 2018 May 9;38(19):4531-4542. doi: 10.1523/JNEUROSCI.2857-17.2018. Epub 2018 Apr 16. J Neurosci. 2018. PMID: 29661964 Free PMC article.
-
Retinal and Nonretinal Contributions to Extraclassical Surround Suppression in the Lateral Geniculate Nucleus.J Neurosci. 2017 Jan 4;37(1):226-235. doi: 10.1523/JNEUROSCI.1577-16.2016. J Neurosci. 2017. PMID: 28053044 Free PMC article.
-
Organization, Function, and Development of the Mouse Retinogeniculate Synapse.Annu Rev Vis Sci. 2020 Sep 15;6:261-285. doi: 10.1146/annurev-vision-121219-081753. Annu Rev Vis Sci. 2020. PMID: 32936733 Review.
Cited by
-
Retinal Neurochemistry.Brain Sci. 2025 Jul 8;15(7):727. doi: 10.3390/brainsci15070727. Brain Sci. 2025. PMID: 40722319 Free PMC article. Review.
-
Design of CMOS-memristor hybrid synapse and its application for noise-tolerant memristive spiking neural network.Front Neurosci. 2025 Mar 5;19:1516971. doi: 10.3389/fnins.2025.1516971. eCollection 2025. Front Neurosci. 2025. PMID: 40109662 Free PMC article.
References
-
- Arakawa H, Suzuki A, Zhao S, Tsytsarev V, Lo FS, Hayashi Y, Itohara S, Iwasato T, Erzurumlu RS. Thalamic NMDA receptor function is necessary for patterning of the thalamocortical somatosensory map and for sensorimotor behaviors. The Journal of Neuroscience. 2014;34:12001–12014. doi: 10.1523/JNEUROSCI.1663-14.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

