Clinical outcomes after CPX-351 in patients with high-risk acute myeloid leukemia: A comparison with a matched cohort from the Spanish PETHEMA registry
- PMID: 37212507
- PMCID: PMC10417130
- DOI: 10.1002/cam4.6120
Clinical outcomes after CPX-351 in patients with high-risk acute myeloid leukemia: A comparison with a matched cohort from the Spanish PETHEMA registry
Abstract
Background: CPX-351 is approved for the treatment of therapy related acute myeloid leukemia (t-AML) and AML with myelodysplastic related changes (MRC-AML). The benefits of this treatment over standard chemotherapy has not been addressed in well matched cohorts of real-life patients.
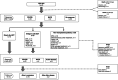
Methods: Retrospective analysis of AML patients treated with CPX-351 as per routine practice. A propensity score matching (PSM) was used to compare their main outcomes with those observed in a matched cohort among 765 historical patients receiving intensive chemotherapy (IC), all of them reported to the PETHEMA epidemiologic registry.
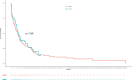
Results: Median age of 79 patients treated with CPX-351 was 67 years old (interquartile range 62-71), 53 were MRC-AML. The complete remission (CR) rate or CR without recovery (CRi) after 1 or 2 cycles of CPX-351 was 52%, 60-days mortality 18%, measurable residual disease <0.1% in 54% (12 out of 22) of them. Stem cell transplant (SCT) was performed in 27 patients (34%), median OS was 10.3 months, and 3-year relapse incidence was 50%. Using PSM, we obtained two comparable cohorts treated with CPX-351 (n = 52) or IC (n = 99), without significant differences in CR/CRi (60% vs. 54%) and median OS (10.3 months vs. 9.1 months), although more patients were bridged to SCT in the CPX-351 group (35% vs. 12%). The results were confirmed when only 3 + 7 patients were included in the historical cohort. In multivariable analyses, SCT was associated with better OS (HR 0.33 95% CI: 0.18-0.59), p < 0.001.
Conclusion: Larger post-authorization studies may provide evidence of the clinical benefits of CPX-351 for AML in the real-life setting.
Keywords: acute myeloid leukemia; clinical observations; intensive chemotherapy; real-world.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Roboz GJ, Larson ML, Rubenstein SE, et al. Final safety and efficacy results from the CPX‐351 early access program for older patients with high‐risk or secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(5):1188‐1194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous