Loss of hepatic PPARα in mice causes hypertension and cardiovascular disease
- PMID: 37212551
- PMCID: PMC10292975
- DOI: 10.1152/ajpregu.00057.2023
Loss of hepatic PPARα in mice causes hypertension and cardiovascular disease
Abstract
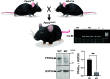
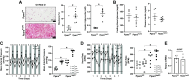
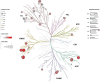
The leading cause of death in patients with nonalcoholic fatty liver disease (NAFLD) is cardiovascular disease (CVD). However, the mechanisms are unknown. Mice deficient in hepatocyte proliferator-activated receptor-α (PPARα) (PparaHepKO) exhibit hepatic steatosis on a regular chow diet, making them prone to manifesting NAFLD. We hypothesized that the PparaHepKO mice might be predisposed to poorer cardiovascular phenotypes due to increased liver fat content. Therefore, we used PparaHepKO and littermate control mice fed a regular chow diet to avoid complications with a high-fat diet, such as insulin resistance and increased adiposity. After 30 wk on a standard diet, male PparaHepKO mice exhibited elevated hepatic fat content compared with littermates as measured by Echo MRI (11.95 ± 1.4 vs. 3.74 ± 1.4%, P < 0.05), hepatic triglycerides (1.4 ± 0.10 vs. 0.3 ± 0.01 mM, P < 0.05), and Oil Red O staining, despite body weight, fasting blood glucose, and insulin levels being the same as controls. The PparaHepKO mice also displayed elevated mean arterial blood pressure (121 ± 4 vs. 108 ± 2 mmHg, P < 0.05), impaired diastolic function, cardiac remodeling, and enhanced vascular stiffness. To determine mechanisms controlling the increase in stiffness in the aorta, we used state-of-the-art PamGene technology to measure kinase activity in this tissue. Our data suggest that the loss of hepatic PPARα induces alterations in the aortas that reduce the kinase activity of tropomyosin receptor kinases and p70S6K kinase, which might contribute to the pathogenesis of NAFLD-induced CVD. These data indicate that hepatic PPARα protects the cardiovascular system through some as-of-yet undefined mechanism.
Keywords: cardiac dysfunction; hepatic steatosis; hypertension; lean NAFLD; nonalcoholic fatty liver disease.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

