Müller's Muscle as a Sensory Proprioceptive Organ: Histological and Histochemical Analysis
- PMID: 37212780
- PMCID: PMC10210510
- DOI: 10.1167/iovs.64.5.18
Müller's Muscle as a Sensory Proprioceptive Organ: Histological and Histochemical Analysis
Abstract
Purpose: The purpose of this study was to determine whether proprioceptive nerves are present in Müller's muscle.
Methods: This was a prospective cohort study in which histologic and immunofluorescence analyses of excised Müller's muscle specimens were performed. Twenty fresh Müller's muscle's specimens from patients undergoing posterior approach ptosis surgery in one center between 2017 and 2018 were evaluated by histologic and immunofluorescent analysis. Axonal types were determined by measuring axon diameter in methylene blue stained plastic sections and by immunofluorescence of frozen sections.
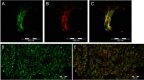
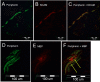
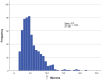
Results: We identified large (greater than 10 microns) and small myelinated fibers in the Müller's muscle, with 6.4% of these fibers being large. Immunofluorescent labeling with choline acetyltransferase showed no evidence of skeletal motor axons in the samples, indicating large axons are likely to be sensory and proprioceptive. In addition, we identified C-fibers using double labeling with peripherin and neural cell adhesion molecules.
Conclusions: Overall, large myelinated sensory fibers are present in the Müller's muscle, likely serving proprioceptive innervation. This suggests that proprioception signals from Müller's muscle may have a role in eyelid spatial positioning and retracting, in addition to visual deprivation. This finding sheds new light on our understanding of this complex mechanism.
Conflict of interest statement
Disclosure:
Figures



References
-
- Rootman DB, Sinha KR, Goldberg RA.. Change in eyelid position following Müller's muscle conjunctival resection with a standard versus variable resection length. Ophthal Plast Reconstr Surg. 2018; 34(4): 355–360. - PubMed
-
- Zauberman NA, Koval T, Kinori M, Matani A, Rosner M, Ben-Simon GJ.. Müller's muscle-conjunctival resection for upper eyelid ptosis: correlation between amount of resected tissue and outcome. Br J Ophthalmol. 2013; 97(4): 408–411. - PubMed
-
- Yuzuriha S, Matsuo K, Hirasawa C, Moriizumi T.. Refined distribution of myelinated trigeminal proprioceptive nerve fibres in Müller's muscle as the mechanoreceptors to induce involuntary reflexive contraction of the levator and frontalis muscles. J Plast Reconstr Aesthetic Surg JPRAS. 2009; 62(11): 1403–1410. - PubMed
-
- Yuzuriha S, Matsuo K, Ishigaki Y, Kikuchi N, Kawagishi K, Moriizumi T.. Efferent and afferent innervations of Müller's muscle related to involuntary contraction of the levator muscle: important for avoiding injury during eyelid surgery. Br J Plast Surg. 2005; 58(1): 42–52. - PubMed
-
- Gasser HS. The classification of nerve fibers. Ohio J Sci. 1941; 41: 145–159.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

