An RNA-based feed-forward mechanism ensures motor switching in oskar mRNA transport
- PMID: 37213090
- PMCID: PMC10202831
- DOI: 10.1083/jcb.202301113
An RNA-based feed-forward mechanism ensures motor switching in oskar mRNA transport
Abstract
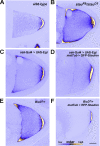
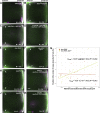
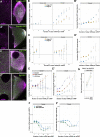
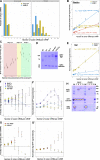
Regulated recruitment and activity of motor proteins is essential for intracellular transport of cargoes, including messenger ribonucleoprotein complexes (RNPs). Here, we show that orchestration of oskar RNP transport in the Drosophila germline relies on interplay between two double-stranded RNA-binding proteins, Staufen and the dynein adaptor Egalitarian (Egl). We find that Staufen antagonizes Egl-mediated transport of oskar mRNA by dynein both in vitro and in vivo. Following delivery of nurse cell-synthesized oskar mRNA into the oocyte by dynein, recruitment of Staufen to the RNPs results in dissociation of Egl and a switch to kinesin-1-mediated translocation of the mRNA to its final destination at the posterior pole of the oocyte. We additionally show that Egl associates with staufen (stau) mRNA in the nurse cells, mediating its enrichment and translation in the ooplasm. Our observations identify a novel feed-forward mechanism, whereby dynein-dependent accumulation of stau mRNA, and thus protein, in the oocyte enables motor switching on oskar RNPs by downregulating dynein activity.
© 2023 Gaspar et al.
Conflict of interest statement
Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. A. Ephrussi reported grants from Deutsche Forschungsgemeinschaft (DFG) during the conduct of the study. No other disclosures were reported.
Figures
Similar articles
-
Tropomyosin 1-I/C coordinates kinesin-1 and dynein motors during oskar mRNA transport.Nat Struct Mol Biol. 2024 Mar;31(3):476-488. doi: 10.1038/s41594-024-01212-x. Epub 2024 Jan 31. Nat Struct Mol Biol. 2024. PMID: 38297086 Free PMC article.
-
Assembly of endogenous oskar mRNA particles for motor-dependent transport in the Drosophila oocyte.Cell. 2009 Nov 25;139(5):983-98. doi: 10.1016/j.cell.2009.10.012. Cell. 2009. Retraction in: Cell. 2010 Oct 29;143(3):485. doi: 10.1016/j.cell.2010.10.011. PMID: 19945381 Retracted.
-
A direct interaction between the RNA-binding proteins Staufen and Tm1-I/C in the oskar mRNA transport complex.Cell Rep. 2025 Jul 22;44(7):115906. doi: 10.1016/j.celrep.2025.115906. Epub 2025 Jun 25. Cell Rep. 2025. PMID: 40570376
-
Swallowing dynein: a missing link in RNA localization?Nat Cell Biol. 2000 Apr;2(4):E60-2. doi: 10.1038/35008687. Nat Cell Biol. 2000. PMID: 10783251 Review.
-
Assembly of mRNA-protein complexes for directional mRNA transport in eukaryotes--an overview.Curr Protein Pept Sci. 2012 Jun;13(4):284-93. doi: 10.2174/138920312801619493. Curr Protein Pept Sci. 2012. PMID: 22708485 Free PMC article. Review.
Cited by
-
The mRNA dynamics underpinning translational control mechanisms of Drosophila melanogaster oogenesis.Biochem Soc Trans. 2024 Oct 30;52(5):2087-2099. doi: 10.1042/BST20231293. Biochem Soc Trans. 2024. PMID: 39263986 Free PMC article. Review.
-
Origin and establishment of the germline in Drosophila melanogaster.Genetics. 2025 Apr 17;229(4):iyae217. doi: 10.1093/genetics/iyae217. Genetics. 2025. PMID: 40180587 Free PMC article. Review.
-
Tropomyosin 1-I/C coordinates kinesin-1 and dynein motors during oskar mRNA transport.Nat Struct Mol Biol. 2024 Mar;31(3):476-488. doi: 10.1038/s41594-024-01212-x. Epub 2024 Jan 31. Nat Struct Mol Biol. 2024. PMID: 38297086 Free PMC article.
-
How germ granules promote germ cell fate.Nat Rev Genet. 2024 Nov;25(11):803-821. doi: 10.1038/s41576-024-00744-8. Epub 2024 Jun 18. Nat Rev Genet. 2024. PMID: 38890558 Review.
-
oskar switches motors halfway through its journey.Nat Rev Mol Cell Biol. 2023 Jul;24(7):451. doi: 10.1038/s41580-023-00620-z. Nat Rev Mol Cell Biol. 2023. PMID: 37253867 No abstract available.
References
-
- Benaglia, T., Chauveau D., Hunter D.R., and Young D.. 2009. Mixtools : An R package for analyzing finite mixture models. J. Stat. Softw. 32. 10.18637/jss.v032.i06 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

