Chronic Obstructive Pulmonary Disease Exacerbations and Pneumonia Hospitalizations Among New Users of Combination Maintenance Inhalers
- PMID: 37213116
- PMCID: PMC10203971
- DOI: 10.1001/jamainternmed.2023.1245
Chronic Obstructive Pulmonary Disease Exacerbations and Pneumonia Hospitalizations Among New Users of Combination Maintenance Inhalers
Abstract
Importance: Clinical guidelines on chronic obstructive pulmonary disease (COPD) recommend inhalers containing long-acting muscarinic antagonists (LAMAs) and long-acting β-agonists (LABAs) over inhalers containing inhaled corticosteroids (ICSs) and LABAs. However, data from randomized clinical trials comparing these combination inhalers (LAMA-LABAs vs ICS-LABAs) have been conflicting and raised concerns of generalizability.
Objective: To assess whether LAMA-LABA therapy is associated with reduced COPD exacerbations and pneumonia hospitalizations compared with ICS-LABA therapy in routine clinical practice.
Design, setting, and participants: This was a 1:1 propensity score-matched cohort study using Optum's Clinformatics Data Mart, a large commercial insurance-claims database. Patients must have had a diagnosis of COPD and filled a new prescription for a combination LAMA-LABA or ICS-LABA inhaler between January 1, 2014, and December 31, 2019. Patients younger than 40 years were excluded, as were those with a prior diagnosis of asthma. The current analysis was performed from February 2021 to March 2023.
Exposures: Combination LAMA-LABA inhalers (aclidinium-formoterol, glycopyrronium-formoterol, glycopyrronium-indacaterol, tiotropium-olodaterol, or umeclidinium-vilanterol) and combination ICS-LABA inhalers (budesonide-formoterol, fluticasone-salmeterol, fluticasone-vilanterol, or mometasone-formoterol).
Main outcome: The primary effectiveness outcome was first moderate or severe COPD exacerbation, and the primary safety outcome was first pneumonia hospitalization. Propensity score matching was used to control for confounding between the 2 groups. Logistic regression analysis was used to estimate propensity scores. Hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards models stratified on matched pairs.
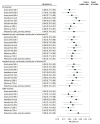
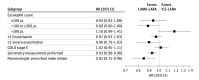
Results: Among 137 833 patients (mean [SD] age, 70.2 [9.9] years; 69 530 [50.4%] female) (107 004 new ICS-LABA users and 30 829 new LAMA-LABA users), 30 216 matched pairs were identified for the primary analysis. Compared with ICS-LABA use, LAMA-LABA use was associated with an 8% reduction in the rate of first moderate or severe COPD exacerbation (HR, 0.92; 95% CI, 0.89-0.96) and a 20% reduction in the rate of first pneumonia hospitalization (HR, 0.80; 95% CI, 0.75-0.86). These findings were robust across a range of prespecified subgroup and sensitivity analyses.
Conclusion: In this cohort study, LAMA-LABA therapy was associated with improved clinical outcomes compared with ICS-LABA therapy, suggesting that LAMA-LABA therapy should be preferred for patients with COPD.
Conflict of interest statement
Figures

References
-
- Global Initiative on Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 report. 2020. Accessed January 23, 2023. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25...
-
- Global Initiative on Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2023 report. 2022, 2023. Accessed January 23, 2023. https://goldcopd.org/wp-content/uploads/2023/01/GOLD-2023-ver-1.2-7Jan20...
-
- Vogelmeier CF, Bateman ED, Pallante J, et al. . Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51-60. doi:10.1016/S2213-2600(12)70052-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

