Safety and Efficacy of Dual Thrombolytic Therapy With Mutant Prourokinase and Small Bolus Alteplase for Ischemic Stroke: A Randomized Clinical Trial
- PMID: 37213122
- PMCID: PMC10203964
- DOI: 10.1001/jamaneurol.2023.1262
Safety and Efficacy of Dual Thrombolytic Therapy With Mutant Prourokinase and Small Bolus Alteplase for Ischemic Stroke: A Randomized Clinical Trial
Abstract
Importance: Dual thrombolytic treatment with small bolus alteplase and mutant prourokinase has the potential to be a safer and more efficacious treatment for ischemic stroke than alteplase alone because mutant prourokinase is designed to act only on degraded fibrin without affecting circulating fibrinogen.
Objective: To assess the safety and efficacy of this dual thrombolytic treatment compared with alteplase.
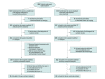
Design, setting, and participants: This controlled, open-label randomized clinical trial with a blinded end point was conducted from August 10, 2019, to March 26, 2022, with a total follow-up of 30 days. Adult patients with ischemic stroke from 4 stroke centers in the Netherlands were enrolled.
Interventions: Patients were randomized (1:1) to receive a bolus of 5 mg of intravenous alteplase and 40 mg of an intravenous infusion of mutant prourokinase (intervention) or usual care with 0.9 mg/kg of intravenous alteplase (control).
Main outcomes and measures: The primary outcome was any intracranial hemorrhage (ICH) on neuroimaging at 24 hours. Secondary outcomes included functional outcome at 30 days, symptomatic ICH, and fibrinogen levels within 24 hours. Analyses were by intention to treat. Treatment effects were adjusted for baseline prognostic factors.
Results: A total of 268 patients were randomized, and 238 (median [IQR] age, 69 [59-77] years; 147 [61.8%] male) provided deferred consent and were included in the intention-to-treat population (121 in the intervention group and 117 in the control group). The median baseline score on the National Institutes of Health Stroke Scale was 3 (IQR, 2-5). Any ICH occurred in 16 of 121 patients (13.2%) in the intervention group and 16 of 117 patients (13.7%) in the control group (adjusted odds ratio, 0.98; 95% CI, 0.46-2.12). Mutant prourokinase led to a nonsignificant shift toward better modified Rankin Scale scores (adjusted common odds ratio, 1.16; 95% CI, 0.74-1.84). Symptomatic ICH occurred in none of the patients in the intervention group and 3 of 117 patients (2.6%) in the control group. Plasma fibrinogen levels at 1 hour remained constant in the intervention group but decreased in the control group (β = 65 mg/dL; 95% CI, 26-105 mg/dL).
Conclusions and relevance: In this trial, dual thrombolytic treatment with small bolus alteplase and mutant prourokinase was found to be safe and did not result in fibrinogen depletion. Further evaluation of thrombolytic treatment with mutant prourokinase in larger trials to improve outcomes in patients with larger ischemic strokes is needed. Overall, in patients with minor ischemic stroke who met indications for treatment with intravenous thrombolytics but were not eligible for treatment with endovascular therapy, dual thrombolytic therapy with intravenous mutant prourokinase was not superior to treatment with intravenous alteplase alone.
Trial registration: ClinicalTrials.gov Identifier: NCT04256473.
Conflict of interest statement
Figures
References
-
- Duvekot MHC, Venema E, Rozeman AD, et al. ; PRESTO Investigators . Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): a prospective observational study. Lancet Neurol. 2021;20(3):213-221. doi:10.1016/S1474-4422(20)30439-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous