Ongoing complete response after treatment cessation with dabrafenib, trametinib, and cetuximab as third-line treatment in a patient with advanced BRAFV600E mutated, microsatellite-stable colon cancer: A case report and literature review
- PMID: 37213293
- PMCID: PMC10196488
- DOI: 10.3389/fonc.2023.1166545
Ongoing complete response after treatment cessation with dabrafenib, trametinib, and cetuximab as third-line treatment in a patient with advanced BRAFV600E mutated, microsatellite-stable colon cancer: A case report and literature review
Abstract
Metastatic BRAFV600E mutated colorectal cancer is associated with poor overall survival and modest effectiveness to standard therapies. Furthermore, survival is influenced by the microsatellite status. Patients with microsatellite-stable and BRAFV600E mutated colorectal cancer have the worst prognosis under the wide range of genetic subgroups in colorectal cancer. Herein, we present a patient case of an impressive therapeutic efficacy of dabrafenib, trametinib, and cetuximab as later-line therapy in a 52-year-old woman with advanced BRAFV600E mutated, microsatellite-stable colon cancer. This patient achieved a complete response after 1 year of triple therapy. Due to skin toxicity grade 3 and recurrent urinary tract infections due to mucosal toxicity, a therapy de-escalation to dabrafenib and trametinib was performed, and the double therapy was administered for further 41 months with ongoing complete response. For 1 year, the patient was off therapy and is still in complete remission.
Keywords: BRAF-V600 mutation; MSS; combination targeted therapy; dabrafenib; trametinib.
Copyright © 2023 Piringer, Decker, Trommet, Kühr, Heibl, Dörfler and Thaler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
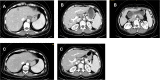
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

