Comparison of the output of a deep learning segmentation model for locoregional breast cancer radiotherapy trained on 2 different datasets
- PMID: 37213441
- PMCID: PMC10199413
- DOI: 10.1016/j.tipsro.2023.100209
Comparison of the output of a deep learning segmentation model for locoregional breast cancer radiotherapy trained on 2 different datasets
Abstract
Introduction: The development of deep learning (DL) models for auto-segmentation is increasing and more models become commercially available. Mostly, commercial models are trained on external data. To study the effect of using a model trained on external data, compared to the same model trained on in-house collected data, the performance of these two DL models was evaluated.
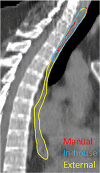
Methods: The evaluation was performed using in-house collected data of 30 breast cancer patients. Quantitative analysis was performed using Dice similarity coefficient (DSC), surface DSC (sDSC) and 95th percentile of Hausdorff Distance (95% HD). These values were compared with previously reported inter-observer variations (IOV).
Results: For a number of structures, statistically significant differences were found between the two models. For organs at risk, mean values for DSC ranged from 0.63 to 0.98 and 0.71 to 0.96 for the in-house and external model, respectively. For target volumes, mean DSC values of 0.57 to 0.94 and 0.33 to 0.92 were found. The difference of 95% HD values ranged 0.08 to 3.23 mm between the two models, except for CTVn4 with 9.95 mm. For the external model, both DSC and 95% HD are outside the range of IOV for CTVn4, whereas this is the case for the DSC found for the thyroid of the in-house model.
Conclusions: Statistically significant differences were found between both models, which were mostly within published inter-observer variations, showing clinical usefulness of both models. Our findings could encourage discussion and revision of existing guidelines, to further decrease inter-observer, but also inter-institute variability.
Keywords: Auto-segmentation; Clinical validation; Deep learning; Loco-regional breast cancer; Radiotherapy.
© 2023 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nienke Bakx received funding from RaySearch Laboratories AB. RaySearch Laboratories AB had no influence on the design of the study but aided with the practical implementation and by offering advice on practical issues.
Figures



References
LinkOut - more resources
Full Text Sources

