Exsolved catalyst particles as a plaything of atmosphere and electrochemistry
- PMID: 37213935
- PMCID: PMC10193834
- DOI: 10.1039/d2ey00036a
Exsolved catalyst particles as a plaything of atmosphere and electrochemistry
Abstract
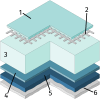
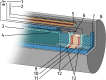
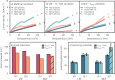
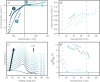
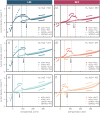
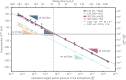
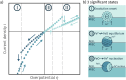
A new type of catalyst preparation yields its active sites not by infiltration but exsolution of reducible transition metals of its own host lattice. These exsolution catalysts offer a high dispersion of catalytically active particles, slow agglomeration, and the possibility of reactivation after poisoning due to redox cycling. The formation of exsolved particles by partial decomposition of the host lattice can be driven by applying a sufficiently reducing atmosphere, elevated temperatures but also by a cathodic bias voltage (provided the host perovskite is an electrode on an oxide ion conducting electrolyte). In addition, such an electrochemical polarisation can change the oxidation state and thus the catalytic activity of exsolved particles. In this work, we investigate the electrochemical switching between an active and an inactive state of iron particles exsolved from thin film mixed conducting model electrodes, namely La0.6Sr0.4FeO3-δ (LSF) and Nd0.6Ca0.4FeO3-δ (NCF), in humid hydrogen atmospheres. We show that the transition between two activity states exhibits a hysteresis-like behaviour in the electrochemical I-V characteristics. Ambient pressure XPS measurements proofed that this hysteresis is linked to the oxidation and reduction of iron particles. Furthermore, it is demonstrated that the surface kinetics of the host material itself has only a negligible impact on the particle exsolution, and that the main impact factors are the surrounding atmosphere as well as the applied electrochemical overpotential. In particular, we suggest a 'kinetic competition' between gas atmosphere and oxygen chemical potential in the mixed conducting electrode and discuss possible ways of how this process takes place.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Babatunde O. M. Munda J. L. Hamam Y. Int. J. Energy Res. 2019;43:6078–6107. doi: 10.1002/er.4388. - DOI
-
- Romanelli F. Eur. Phys. J. Plus. 2016;131:53. doi: 10.1140/epjp/i2016-16053-3. - DOI
-
- Zsiborács H. Baranyai N. H. Vincze A. Zentkó L. Birkner Z. Máté K. Pintér G. Electronics. 2019;8:729. doi: 10.3390/electronics8070729. - DOI
-
- Moriarty P. Honnery D. Int. J. Hydrogen Energy. 2007;32:1616–1624. doi: 10.1016/j.ijhydene.2006.12.008. - DOI
-
- Ebbesen S. D. Mogensen M. J. Power Sources. 2009;193:349–358. doi: 10.1016/j.jpowsour.2009.02.093. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
