Modeling Pkd1 gene-targeted strategies for correction of polycystic kidney disease
- PMID: 37214311
- PMCID: PMC10199405
- DOI: 10.1016/j.omtm.2023.03.016
Modeling Pkd1 gene-targeted strategies for correction of polycystic kidney disease
Expression of concern in
-
Modeling Pkd1 gene-targeted strategies for correction of polycystic kidney disease.Mol Ther Methods Clin Dev. 2025 Jan 28;33(1):101422. doi: 10.1016/j.omtm.2025.101422. eCollection 2025 Mar 13. Mol Ther Methods Clin Dev. 2025. PMID: 39944270 Free PMC article. No abstract available.
Abstract
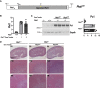
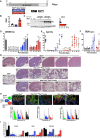
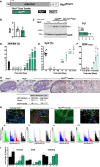
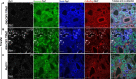
Autosomal dominant polycystic kidney disease (ADPKD) causes renal cysts and leads to end-stage renal disease in midlife due mainly to PKD1 gene mutations. Virtually no studies have explored gene therapeutic strategies for long-term effective treatment of PKD. Toward this aim, the severely cystic Pkd1-null mouse model was targeted with a series of transgene transfers using genomic Pkd1 under its regulatory elements (Pkd1wt), a kidney-targeted Pkd1 gene (SBPkd1), or Pkd1Minigene. The introduced Pkd1wt gene constructs with ∼8-fold overexpression display similar endogenous cellular profiles and full complementation of Pkd1-/- phenotype and establish the referral Pkd1 genomic length for proper regulation. SBPkd1 transgene transfer expressing 0.6- or 7-fold Pkd1 endogenous levels is sufficient to correct glomerular and proximal tubular cysts and to markedly postpone cysts in other tubular segments as well, showing that the small SB elements appreciably overlap with Pkd1 promoter/5' UTR regulation. Renal-targeted Pkd1Minigene at high copy numbers conveys an expression level similar to that of the endogenous Pkd1 gene, with widespread and homogeneous weak Pkd1 cellular signal, partially rescuing all cystic tubular segments. These transgene transfers determine that Pkd1 intragenic sequences regulate not only expression levels but also spatiotemporal patterns. Importantly, our study demonstrates that Pkd1 re-expression from hybrid therapeutic constructs can ameliorate, with considerably extended lifespan, or eliminate PKD.
Keywords: PKD mouse models; PKD transcriptional regulation; Pc1; Pkd1 RNAscope; Pkd1 gene therapy; Pkd1 locus/minigene; polycystic kidney disease; polycystin; small SB renal cassette.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Michaud J., Russo P., Grignon A., Dallaire L., Bichet D., Rosenblatt D., Lamothe E., Lambert M. Autosomal dominant polycystic kidney disease in the fetus. Am. J. Med. Genet. 1994;51:240–246. - PubMed
-
- Novelli G., Frontali M., Baldini D., Bosman C., Dallapiccola B., Pachì A., Torcia F. Prenatal diagnosis of adult polycystic kidney disease with DNA markers on chromosome 16 and the genetic heterogeneity problem. Prenat. Diagn. 1989;9:759–767. - PubMed
-
- Gal A., Wirth B., Kääriäinen H., Lucotte G., Landais P., Gillessen-Kaesbach G., Müller-Wiefel D.E., Zerres K. Childhood manifestation of autosomal dominant polycystic kidney disease: no evidence for genetic heterogeneity. Clin. Genet. 1989;35:13–19. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

