Stereoselective glycosylation reactions with 2-deoxyglucose: a computational study of some catalysts
- PMID: 37214423
- PMCID: PMC10195097
- DOI: 10.1016/j.comptc.2023.114122
Stereoselective glycosylation reactions with 2-deoxyglucose: a computational study of some catalysts
Abstract
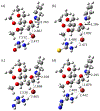
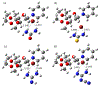
2-Deoxy glycosides are important components of many oligosaccharides with antibiotic and anti-cancer activity, but their synthesis can be very challenging. Phenanthrolines and substituted pyridines promote stereoselective glycosylation of 1-bromo sugars via a double SN2 mechanism. Pyridine reacting with α-bromo, 2-deoxyglucose was chosen to model this reaction. The first step involves displacement of bromide by pyridine which can be rate limiting because bromide ion is poorly solvated in the non-polar solvents used for these reactions. We examined a series of small molecules to bind bromide and stabilize this transition state. Geometry optimization and vibrational frequencies were calculated using M06-2X/6-31+G(d,p) and SMD implicit solvation for diethyl ether. More accurate energies were obtained with M06-2X/aug-cc-pVTZ and implicit solvation. Urea, thiourea, guanidine and cyanoguanidine bind bromide more strongly than alkylamines, (NH2CH2CH2)nNH3-n. Compared to the uncatalyzed reaction, urea, thiourea and cyanoguanidine lower the free energy of the transition state by 3 kcal/mol while guanidine lowers the barrier by 2 kcal/mol.
Keywords: 2-deoxyglucose; DFT; catalysis; glycosylation.
Conflict of interest statement
Conflict of Interest Statement The authors declare that they have no known conflict of interests that could have appeared to influence the work reported in this paper. Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
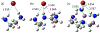





References
-
- Nicolaou KC, Li YW, Sugita K, Monenschein H, Guntupalli P, Mitchell HJ, Fylaktakidou KC, Vourloumis D, Giannakakou P, O’Brate A, Total synthesis of apoptolidin: Completion of the synthesis and analogue synthesis and evaluation, J. Am. Chem. Soc, 125 (2003) 15443–15454. - PubMed
-
- Zhu XM, Schmidt RR, New Principles for Glycoside-Bond Formation, Angew. Chem. Int. Ed. Engl, 48 (2009) 1900–1934. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
