Hydroxychloroquine and azithromycin alter the contractility of living porcine heart slices
- PMID: 37214466
- PMCID: PMC10196358
- DOI: 10.3389/fphar.2023.1127388
Hydroxychloroquine and azithromycin alter the contractility of living porcine heart slices
Abstract
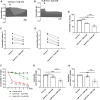
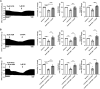
The cardiotoxicity risk of hydroxychloroquine (HCQ) and azithromycin (AZM) has been the subject of intensive research triggered by safety concerns in COVID-19 patients. HCQ and AZM have been associated with QT interval prolongation and drug-induced arrhythmias, however other cardiotoxicity mechanisms remain largely unexplored. Our group has pioneered the living heart slice preparation, an ex-vivo platform that maintains native cardiac tissue architecture and physiological electrical and contractile properties. Here, we evaluated the cardiotoxic effect of HCQ and AZM applied alone or in combination on cardiac contractility by measuring contractile force and contraction kinetics in heart slices prepared from porcine hearts. Our results show that clinically relevant concentrations of HCQ monotherapy (1-10 µM) reduced contractile force and contraction kinetics in porcine slices in a dose-dependent manner. However, AZM monotherapy decreased contractile force and contraction kinetics only at higher concentrations (30 µM). Combination of HCQ and AZM induced a dose-dependent effect similar to HCQ alone. Furthermore, pre-treating porcine heart slices with the L-type calcium channel agonist Bay K8644 prevented the effect of both drugs, while administration of Bay K8644 after drugs interventions largely reversed the effects, suggesting a mechanism involving inhibition of L-type calcium channels. These findings indicate that HCQ and AZM alter cardiac function beyond QT prolongation with significant contractile dysfunction in intact cardiac tissue. Our porcine heart slices provide a powerful platform to investigate mechanisms of drug cardiotoxicity.
Keywords: Bay K8644; COVID-19; calcium channels; cardiotoxicity; myocardial slices; organotypic ex-vivo models; safety pharmacology.
Copyright © 2023 Wu, Ross, Ipek, Thompson, Johnson, Wu and Camelliti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Adverse effects of hydroxychloroquine and azithromycin on contractility and arrhythmogenicity revealed by human engineered cardiac tissues.J Mol Cell Cardiol. 2021 Apr;153:106-110. doi: 10.1016/j.yjmcc.2020.12.014. Epub 2020 Dec 27. J Mol Cell Cardiol. 2021. PMID: 33373642 Free PMC article.
-
System biological investigations of hydroxychloroquine and azithromycin targets and their implications in QT interval prolongation.Chem Biol Interact. 2020 Dec 1;332:109299. doi: 10.1016/j.cbi.2020.109299. Epub 2020 Oct 22. Chem Biol Interact. 2020. PMID: 33098839 Free PMC article.
-
The impact of hydroxychloroquine-azithromycin combination on Tpeak-to-end and Tpeak-to-end/QT ratio during a short treatment course.Ann Noninvasive Electrocardiol. 2021 Jul;26(4):e12846. doi: 10.1111/anec.12846. Epub 2021 May 6. Ann Noninvasive Electrocardiol. 2021. PMID: 33956361 Free PMC article.
-
A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment.Sci Rep. 2020 Dec 17;10(1):22139. doi: 10.1038/s41598-020-77748-x. Sci Rep. 2020. PMID: 33335141 Free PMC article.
-
Chloroquine and hydroxychloroquine for COVID-19: Perspectives on their failure in repurposing.J Clin Pharm Ther. 2021 Feb;46(1):17-27. doi: 10.1111/jcpt.13267. Epub 2020 Sep 27. J Clin Pharm Ther. 2021. PMID: 32981089 Free PMC article. Review.
Cited by
-
A novel method to extend viability and functionality of living heart slices.Front Cardiovasc Med. 2023 Oct 10;10:1244630. doi: 10.3389/fcvm.2023.1244630. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37881724 Free PMC article.
References
-
- Capel R. A., Herring N., Kalla M., Yavari A., Mirams G. R., Douglas G., et al. (2015). Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: Novel electrophysiological insights and therapeutic potential. Heart rhythm. 12 (10), 2186–2194. 10.1016/j.hrthm.2015.05.027 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

