Do people with hereditary cancer syndromes inform their at-risk relatives? A systematic review and meta-analysis
- PMID: 37214514
- PMCID: PMC10194207
- DOI: 10.1016/j.pecinn.2023.100138
Do people with hereditary cancer syndromes inform their at-risk relatives? A systematic review and meta-analysis
Abstract
Purpose: To evaluate rates of familial disclosure of hereditary cancer syndrome information.
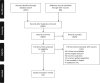
Methods: A systematic review and meta-analysis was conducted in accordance with PRISMA guidelines (PROSPERO no.: CRD42020134276). Key electronic databases were searched to identify studies evaluating hereditary cancer syndrome cascade relative disclosure. Eligible studies were subjected to meta-analysis.
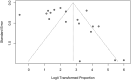
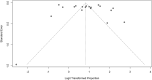
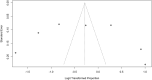
Results: Thirty-four studies met inclusion criteria. Among 11,711 included relatives, 70% (95% CI 60 - 78%) were informed of their risk of carrying a cancer-associated pathogenic variant; of 2,875 relatives informed of their risk who were evaluated for uptake of cascade testing, 43% (95% CI 27 - 61%) completed testing. Rates of disclosure were higher among female vs male relatives (79% [95% CI 73% - 84%] vs 67% [95% CI 57% - 75%]) and first-degree vs second-degree relatives (83% [95% CI 77% - 88%] vs 58% [95% CI 45 - 69%]).
Conclusion: Nearly one-third of at-risk relatives remain uninformed of their risk of carrying a cancer-associated pathogenic variant. Even among those informed, fewer than half subsequently complete genetic testing, representing a critical missed opportunity for precision cancer prevention.
Innovation: Five studies evaluating interventions to improve disclosure rates were generally ineffective. Urgent work is needed to elucidate barriers to relative disclosure by probands to develop targeted interventions that can optimize proband-mediated cascade genetic testing rates.
Keywords: Cascade genetic testing; Disclosure; Hereditary breast and ovarian cancer; Hereditary cancer syndromes; Lynch syndrome.
© 2023 The Authors.
Conflict of interest statement
Kevin Holcomb reports a relationship with Johnson & Johnson that includes: consulting or advisory.
Figures



Similar articles
-
Direct letters to relatives at risk of hereditary cancer-study protocol for a multi-center randomized controlled trial of healthcare-assisted versus family-mediated risk disclosure at Swedish cancer genetics clinics (DIRECT-study).Trials. 2023 Dec 17;24(1):810. doi: 10.1186/s13063-023-07829-5. Trials. 2023. PMID: 38105176 Free PMC article.
-
Prophylactic Oophorectomy: Reducing the U.S. Death Rate from Epithelial Ovarian Cancer. A Continuing Debate.Oncologist. 1996;1(5):326-330. Oncologist. 1996. PMID: 10388011
-
Uptake of Cascade Genetic Testing for Hereditary Breast and Ovarian Cancer: A Systematic Review and Meta-Analysis.Clin Obstet Gynecol. 2024 Dec 1;67(4):702-710. doi: 10.1097/GRF.0000000000000895. Epub 2024 Oct 18. Clin Obstet Gynecol. 2024. PMID: 39431491
-
Communication about positive BRCA1 and BRCA2 genetic test results and uptake of testing in relatives in a diverse Asian setting.J Genet Couns. 2021 Jun;30(3):720-729. doi: 10.1002/jgc4.1360. Epub 2020 Nov 27. J Genet Couns. 2021. PMID: 33245177
-
The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice.Fam Cancer. 2019 Jan;18(1):127-135. doi: 10.1007/s10689-018-0089-z. Fam Cancer. 2019. PMID: 29846880
Cited by
-
Liminality between direct and family-mediated contact in the communication of genetic information to at-risk relatives.Eur J Hum Genet. 2024 May;32(5):477-478. doi: 10.1038/s41431-024-01605-y. Epub 2024 Apr 16. Eur J Hum Genet. 2024. PMID: 38627543 Free PMC article. No abstract available.
-
Addressing family communication in genetic counseling: A scoping review of process studies.J Genet Couns. 2025 Aug;34(4):e70067. doi: 10.1002/jgc4.70067. J Genet Couns. 2025. PMID: 40801425 Free PMC article. Review.
-
Genomic findings with familial implications: agenda setting in light of mainstreaming.Open Res Eur. 2025 Jan 10;5:4. doi: 10.12688/openreseurope.19128.1. eCollection 2025. Open Res Eur. 2025. PMID: 40212820 Free PMC article.
-
Cascade genetic testing in hereditary cancer: exploring the boundaries of the Italian legal framework.Fam Cancer. 2024 Nov 20;24(1):9. doi: 10.1007/s10689-024-00430-y. Fam Cancer. 2024. PMID: 39565467
-
The experience of receiving a letter from a cancer genetics clinic about risk for hereditary cancer.Eur J Hum Genet. 2024 May;32(5):539-544. doi: 10.1038/s41431-024-01551-9. Epub 2024 Feb 14. Eur J Hum Genet. 2024. PMID: 38355958 Free PMC article.
References
-
- Li X., et al. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin Cancer Res. 2016;22(15):3971–3981. - PubMed
-
- Engel C., et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol. 2010;8(2):174–182. - PubMed
-
- de Jong A.E., et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130(3):665–671. - PubMed
-
- Daly M.B., et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77–102. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

