Cytotoxic Evaluation in HaCaT Cells of the Pa.7 Bacteriophage from Cutibacterium (Propionibacterium) acnes, Free and Encapsulated Within Liposomes
- PMID: 37214651
- PMCID: PMC10196082
- DOI: 10.1089/phage.2022.0038
Cytotoxic Evaluation in HaCaT Cells of the Pa.7 Bacteriophage from Cutibacterium (Propionibacterium) acnes, Free and Encapsulated Within Liposomes
Abstract
Introduction: Acne is a multifactorial disease involving the colonization of skin follicles by Cutibacterium (formerly Propionibacterium) acnes. A combination of different retinoid-derived products, antibiotics, and hormonal antiandrogens are used to treat the disease, but these treatments require extended periods, may have secondary effects, are expensive, and not always effective. Owing to antibiotic resistance, the use of bacteriophages has been proposed as an alternative treatment. However, if they are intended for a cosmetic or pharmaceutical use, it is necessary to evaluate the safety of the phages and the preparations containing them.
Materials and methods: In this study, the cytotoxicity of Pa.7 bacteriophage was evaluated in HaCaT cells, along with a liposome suitable for their encapsulation, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and trypan blue assays.
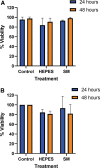
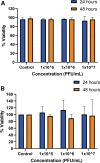
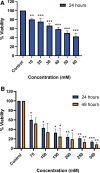
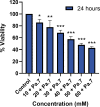
Results: We found that Pa.7 was not cytotoxic for HaCaT cells. Also, 30 mM of liposomes, or below are considered noncytotoxic concentrations.
Conclusion: Phages encapsulated in the liposomes presented in this study can be used safely for skin treatments.
Keywords: Cutibacterium acnes; acne; cytotoxicity; liposomes; phage therapy.
Copyright 2023, Mary Ann Liebert, Inc., publishers.
Conflict of interest statement
M.J.V. declares she was in the past a member of the spin-off company SciPhage S.A.S., which works for the development of phage therapy in Colombia. She is also among the inventors of the patent no. NC2018/0008178, entitled “Composicionestópicas que comprendenbacteriófagos que se encapsulanenliposomas” (Topical compositions comprising bacteriophages encapsulated in liposomes). Other authors declare no competing interests.
Figures

References
-
- Titus S, Hodge J. Diagnosis and treatment of acne. Am Fam Physician 2012;86(8):734–740. - PubMed
LinkOut - more resources
Full Text Sources
