Quinolone-3-amidoalkanol: A New Class of Potent and Broad-Spectrum Antimicrobial Agent
- PMID: 37214682
- PMCID: PMC10193574
- DOI: 10.1021/acsomega.3c01406
Quinolone-3-amidoalkanol: A New Class of Potent and Broad-Spectrum Antimicrobial Agent
Abstract
Herein, we describe 39 novel quinolone compounds bearing a hydrophilic amine chain and varied substituted benzyloxy units. These compounds demonstrate broad-spectrum activities against acid-fast bacterium, Gram-positive and -negative bacteria, fungi, and leishmania parasite. Compound 30 maintained antitubercular activity against moxifloxacin-, isoniazid-, and rifampicin-resistant Mycobacterium tuberculosis, while 37 exhibited low micromolar activities (<1 μg/mL) against World Health Organization (WHO) critical pathogens: Cryptococcus neoformans, Acinetobacter baumannii, and Pseudomonas aeruginosa. Compounds in this study are metabolically robust, demonstrating % remnant of >98% after 30 min in the presence of human, rat, and mouse liver microsomes. Several compounds thus reported here are promising leads for the treatment of diseases caused by infectious agents.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
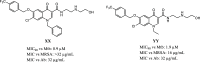

References
-
- World Health Organization . Tuberculosis; World Health Organization: Geneva, Switzerland, 2022. https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
Grants and funding
LinkOut - more resources
Full Text Sources

