Reactions of Methyl Radicals with Aniline Acting as Hydrogen Donors and as Methyl Radical Acceptors
- PMID: 37214701
- PMCID: PMC10193547
- DOI: 10.1021/acsomega.3c01029
Reactions of Methyl Radicals with Aniline Acting as Hydrogen Donors and as Methyl Radical Acceptors
Abstract
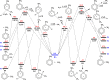
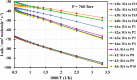
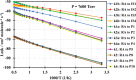
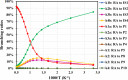
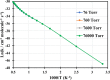
The present investigation theoretically reports the comprehensive kinetic mechanism of the reaction between aniline and the methyl radical over a wide range of temperatures (300-2000 K) and pressures (76-76,000 Torr). The potential energy surface of the C6H5NH2 + CH3 reaction has been established at the CCSD(T)//M06-2X/6-311++G(3df,2p) level of theory. The conventional transition-state theory (TST) was utilized to calculate rate constants for the elementary reaction channels, while the stochastic RRKM-based master equation framework was applied for the T- and P-dependent rate-coefficient calculation of multiwell reaction paths. Hindered internal rotation and Eckart tunneling treatments were included. The H-abstraction from the -NH2 group of aniline (to form P1 (C6H5NH + CH4)) has been found to compete with the CH3-addition on the C atom at the ortho site of aniline (to form IS2) with the atmospheric rate expressions (in cm3 molecule-1 s-1) as ka1 = 7.5 × 10-23 T3.04 exp[(-40.63 ± 0.29 kJ·mol-1)/RT] and kb2 = 2.29 × 10-3 T-3.19 exp[(-56.94 ± 1.17 kJ·mol-1)/RT] for T = 300-2000 K and P = 760 Torr. Even though rate constants of several reaction channels decrease with increasing pressures, the total rate constant ktotal = 7.71 × 10-17 T1.20 exp[(-40.96 ± 2.18 kJ·mol-1)/RT] of the title reaction still increases as the pressure increases in the range of 76-76,000 Torr. The calculated enthalpy changes for some species are in good agreement with the available experimental data within their uncertainties (the maximum deviation between theory and experiment is ∼11 kJ·mol-1). The T1 diagnostic and spin contamination analysis for all species involved have also been observed. This work provides sound quality rate coefficients for the title reaction, which will be valuable for the development of detailed combustion reaction mechanisms for hydrocarbon fuels.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Anjalin M.; Kanagathara N.; Suganthi A. R. B. A brief review on aniline and its derivatives. Mater. Today: Proc. 2020, 33, 4751–4755. 10.1016/j.matpr.2020.08.358. - DOI
-
- Shahrezaei F.; Mansouri Y.; Zinatizadeh A. A. L.; Akhbari A. Photocatalytic Degradation of Aniline Using TiO2 Nanoparticles in a Vertical Circulating Photocatalytic Reactor. Int. J. Photoenergy 2012, 2012, 1–8. 10.1155/2012/430638. - DOI
LinkOut - more resources
Full Text Sources

