This is a preprint.
The stabilized supralinear network accounts for the contrast dependence of visual cortical gamma oscillations
- PMID: 37214812
- PMCID: PMC10197697
- DOI: 10.1101/2023.05.11.540442
The stabilized supralinear network accounts for the contrast dependence of visual cortical gamma oscillations
Update in
-
The stabilized supralinear network accounts for the contrast dependence of visual cortical gamma oscillations.PLoS Comput Biol. 2024 Jun 27;20(6):e1012190. doi: 10.1371/journal.pcbi.1012190. eCollection 2024 Jun. PLoS Comput Biol. 2024. PMID: 38935792 Free PMC article.
Abstract
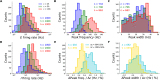
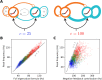
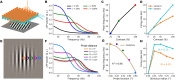
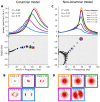
When stimulated, neural populations in the visual cortex exhibit fast rhythmic activity with frequencies in the gamma band (30-80 Hz). The gamma rhythm manifests as a broad resonance peak in the power-spectrum of recorded local field potentials, which exhibits various stimulus dependencies. In particular, in macaque primary visual cortex (V1), the gamma peak frequency increases with increasing stimulus contrast. Moreover, this contrast dependence is local: when contrast varies smoothly over visual space, the gamma peak frequency in each cortical column is controlled by the local contrast in that column's receptive field. No parsimonious mechanistic explanation for these contrast dependencies of V1 gamma oscillations has been proposed. The stabilized supralinear network (SSN) is a mechanistic model of cortical circuits that has accounted for a range of visual cortical response nonlinearities and contextual modulations, as well as their contrast dependence. Here, we begin by showing that a reduced SSN model without retinotopy robustly captures the contrast dependence of gamma peak frequency, and provides a mechanistic explanation for this effect based on the observed non-saturating and supralinear input-output function of V1 neurons. Given this result, the local dependence on contrast can trivially be captured in a retinotopic SSN which however lacks horizontal synaptic connections between its cortical columns. However, long-range horizontal connections in V1 are in fact strong, and underlie contextual modulation effects such as surround suppression. We thus explored whether a retinotopically organized SSN model of V1 with strong excitatory horizontal connections can exhibit both surround suppression and the local contrast dependence of gamma peak frequency. We found that retinotopic SSNs can account for both effects, but only when the horizontal excitatory projections are composed of two components with different patterns of spatial fall-off with distance: a short-range component that only targets the source column, combined with a long-range component that targets columns neighboring the source column. We thus make a specific qualitative prediction for the spatial structure of horizontal connections in macaque V1, consistent with the columnar structure of cortex.
Figures



References
-
- Anderson J., Lampl I., Gillespie D., and Ferster D. (2000). The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science (New York, NY), 290(5498):1968–1972. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
