This is a preprint.
Crucial neuroprotective roles of the metabolite BH4 in dopaminergic neurons
- PMID: 37214873
- PMCID: PMC10197517
- DOI: 10.1101/2023.05.08.539795
Crucial neuroprotective roles of the metabolite BH4 in dopaminergic neurons
Abstract
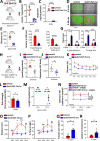
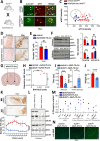
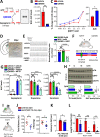
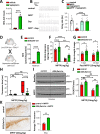
Dopa-responsive dystonia (DRD) and Parkinson's disease (PD) are movement disorders caused by the dysfunction of nigrostriatal dopaminergic neurons. Identifying druggable pathways and biomarkers for guiding therapies is crucial due to the debilitating nature of these disorders. Recent genetic studies have identified variants of GTP cyclohydrolase-1 (GCH1), the rate-limiting enzyme in tetrahydrobiopterin (BH4) synthesis, as causative for these movement disorders. Here, we show that genetic and pharmacological inhibition of BH4 synthesis in mice and human midbrain-like organoids accurately recapitulates motor, behavioral and biochemical characteristics of these human diseases, with severity of the phenotype correlating with extent of BH4 deficiency. We also show that BH4 deficiency increases sensitivities to several PD-related stressors in mice and PD human cells, resulting in worse behavioral and physiological outcomes. Conversely, genetic and pharmacological augmentation of BH4 protects mice from genetically- and chemically induced PD-related stressors. Importantly, increasing BH4 levels also protects primary cells from PD-affected individuals and human midbrain-like organoids (hMLOs) from these stressors. Mechanistically, BH4 not only serves as an essential cofactor for dopamine synthesis, but also independently regulates tyrosine hydroxylase levels, protects against ferroptosis, scavenges mitochondrial ROS, maintains neuronal excitability and promotes mitochondrial ATP production, thereby enhancing mitochondrial fitness and cellular respiration in multiple preclinical PD animal models, human dopaminergic midbrain-like organoids and primary cells from PD-affected individuals. Our findings pinpoint the BH4 pathway as a key metabolic program at the intersection of multiple protective mechanisms for the health and function of midbrain dopaminergic neurons, identifying it as a potential therapeutic target for PD.
Keywords: Dopamine; GCH1; LRRK2; PRKN; ROS; ferroptosis; mitochondria; sepiapterin.
Conflict of interest statement
Declaration of interest The authors declare no conflict of interest.
Figures


References
-
- Amboni M., Cozzolino A., Longo K., Picillo M., and Barone P. (2008). Freezing of gait and executive functions in patients with Parkinson’s disease. Mov. Disord. - PubMed
-
- Bandres-Ciga S., Saez-Atienzar S., Kim J.J., Makarious M.B., Faghri F., Diez-Fairen M., Iwaki H., Leonard H., Botia J., Ryten M., et al. (2020). Large-scale pathway specific polygenic risk and transcriptomic community network analysis identifies novel functional pathways in Parkinson disease. Acta Neuropathol. 140. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
