This is a preprint.
Cardiomyocyte Targeting Peptide to Deliver Amiodarone
- PMID: 37214919
- PMCID: PMC10197706
- DOI: 10.1101/2023.05.10.540206
Cardiomyocyte Targeting Peptide to Deliver Amiodarone
Update in
-
Cardiomyocyte-Targeting Peptide to Deliver Amiodarone.Pharmaceutics. 2023 Aug 9;15(8):2107. doi: 10.3390/pharmaceutics15082107. Pharmaceutics. 2023. PMID: 37631321 Free PMC article.
Abstract
Background: Amiodarone is underutilized due to significant off-target toxicities. We hypothesized that targeted delivery to the heart would lead to lowering of dose by utilizing a cardiomyocyte targeting peptide (CTP), a cell penetrating peptide identified by our prior phage display work.
Methods: CTP was synthesized thiolated at the N-terminus, conjugated to amiodarone via Schiff base chemistry, HPLC purified and confirmed with MALDI/TOF. Stability of the conjugate was assessed using serial HPLCs. Guinea pigs (GP) were injected intraperitoneally daily with vehicle (7 days), amiodarone (7 days; 80mg/Kg), CTP-amiodarone (5 days;26.3mg/Kg), or CTP (5 days; 17.8mg/Kg), after which GPs were euthanized, hearts excised, perfused on a Langendorff apparatus with Tyrode's solution and blebbistatin (5μM) to minimize contractions. Voltage (RH237) and Ca 2+ -indicator dye (Rhod-2/AM) were injected, fluorescence from the epicardium split and focused on two cameras capturing at 570-595nm for cytosolic Ca 2+ and 610-750nm wavelengths for voltage. Subsequently, hearts were paced at 250ms with programmed stimulation to measure changes in conduction velocities (CV), action potential duration (APD) and Ca 2+ transient durations at 90% recovery (CaTD 90 ). mRNA was extracted from all hearts and RNA sequencing performed with results compared to control hearts.
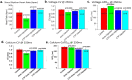
Results: CTP-amiodarone remained stable for up to 21 days at 37°C. At ∼1/15 th of the dose of amiodarone, CTP-amiodarone decreased CV in hearts significantly compared to control GPs (0.92±0.05 vs. 1.00±0.03m/s, p=0.0007), equivalent to amiodarone alone (0.87±0.08ms, p=0.0003). Amiodarone increased APD (192±5ms vs. 175±8ms for vehicle, p=0.0025), while CTP-amiodarone decreased it significantly (157±16ms, p=0.0136) similar to CTP alone (155±13ms, p=0.0039). Both amiodarone and CTP-amiodarone significantly decreased calcium transients compared to controls. CTP-amiodarone and CTP decreased CaTD 90 to an extent greater than amiodarone alone (p<0.001). RNA-seq showed that CTP alone increased the expression of DHPR and SERCA2a, while decreasing expression of proinflammatory genes NF-kappa B, TNF-α, IL-1β, and IL-6.
Conclusions: Our data suggests that CTP can deliver amiodarone to cardiomyocytes at ∼1/15 th the total molar dose of amiodarone needed to produce comparable slowing of CVs. The ability of CTP to decrease AP durations and CaTD 90 may be related to its increase in expression of Ca-handling genes, and merits further study.
Figures






Similar articles
-
Cardiomyocyte-Targeting Peptide to Deliver Amiodarone.Pharmaceutics. 2023 Aug 9;15(8):2107. doi: 10.3390/pharmaceutics15082107. Pharmaceutics. 2023. PMID: 37631321 Free PMC article.
-
Chronic in vivo and in vitro effects of amiodarone on guinea pig hearts.J Pharmacol Exp Ther. 1996 Aug;278(2):906-12. J Pharmacol Exp Ther. 1996. PMID: 8768746
-
Conjugation of amiodarone to a novel cardiomyocyte cell penetrating peptide for potential targeted delivery to the heart.Front Chem. 2023 Jul 12;11:1220573. doi: 10.3389/fchem.2023.1220573. eCollection 2023. Front Chem. 2023. PMID: 37547910 Free PMC article.
-
Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent.Am J Cardiol. 1999 Nov 4;84(9A):20R-28R. doi: 10.1016/s0002-9149(99)00698-0. Am J Cardiol. 1999. PMID: 10568656 Review.
-
Acute and chronic effects of amiodarone on mammalian ventricular cells.Jpn Heart J. 1996 Sep;37(5):719-30. doi: 10.1536/ihj.37.719. Jpn Heart J. 1996. PMID: 8973384 Review.
References
-
- Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 - DOI - PMC - PubMed
-
- Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693 - DOI - PMC - PubMed
-
- Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns H, Eckardt L, Gessler N, Goette A, Haegeli LM, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2022;43:1219–1230. doi: 10.1093/eurheartj/ehab593 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
