This is a preprint.
Dimeric Transmembrane Structure of the SARS-CoV-2 E Protein
- PMID: 37214926
- PMCID: PMC10197518
- DOI: 10.1101/2023.05.07.539752
Dimeric Transmembrane Structure of the SARS-CoV-2 E Protein
Update in
-
Dimeric Transmembrane Structure of the SARS-CoV-2 E Protein.Commun Biol. 2023 Nov 1;6(1):1109. doi: 10.1038/s42003-023-05490-x. Commun Biol. 2023. PMID: 37914906 Free PMC article.
Abstract
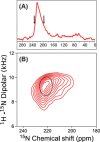
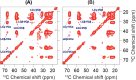
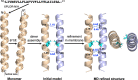
The SARS-CoV-2 E protein is a transmembrane (TM) protein with its N-terminus exposed on the external surface of the virus. Here, the TM structure of the E protein is characterized by oriented sample and magic angle spinning solid-state NMR in lipid bilayers and refined by molecular dynamics simulations. This protein has been found to be a pentamer, with a hydrophobic pore that appears to function as an ion channel. We identified only a symmetric helix-helix interface, leading to a dimeric structure that does not support channel activity. The two helices have a tilt angle of only 6°, resulting in an extended interface dominated by Leu and Val sidechains. While residues Val14-Thr35 are almost all buried in the hydrophobic region of the membrane, Asn15 lines a water-filled pocket that potentially serves as a drug-binding site. The E and other viral proteins may adopt different oligomeric states to help perform multiple functions.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



References
-
- Organization, W.H. (Accessed April 27, 2023).
-
- Aronin S.I. & Sadigh M. Severe acute respiratory syndrome. Conn Med 68, 207–215 (2004). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
