This is a preprint.
Immunogenicity of NVX-CoV2373 in PREVENT-19: A Phase 3, Randomized, Placebo-Controlled Trial in Adults in the United States and Mexico
- PMID: 37214968
- PMCID: PMC10197803
- DOI: 10.1101/2023.05.08.23289670
Immunogenicity of NVX-CoV2373 in PREVENT-19: A Phase 3, Randomized, Placebo-Controlled Trial in Adults in the United States and Mexico
Abstract
Background: NVX-CoV2373, an adjuvanted, recombinant SARS-CoV-2 spike (rS) protein vaccine, consistently demonstrated protective efficacy against COVID-19 in clinical trials and has received regulatory authorizations or approvals worldwide.
Methods: PREVENT-19 (NCT04611802) is a phase 3, randomized, observer-blinded, placebo-controlled trial evaluating safety, immunogenicity, and efficacy of NVX-CoV2373 in ≈30 000 participants ≥18 years in the United States and Mexico. Vaccine humoral immune response (ie, serum immunoglobulin [IgG] antibodies, hACE2 receptor binding inhibition antibodies, and neutralizing antibodies to SARS-CoV-2) (ancestral strain) was assessed in 1200 participants randomly selected and equally divided between participants 18-64 and ≥65 years.
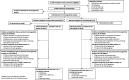
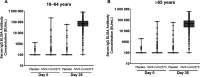
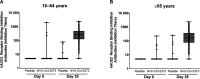
Results: In the per protocol analysis, NVX-CoV2373 induced vigorous serum antibody responses among the 1063 analyzed participants who were SARS-CoV-2 seronegative at baseline, received both doses of study treatment, and had serology results available 2 weeks after dose 2. Geometric mean (GM) responses in both younger and older adults were higher among recipients of vaccine versus placebo for IgG (64 259 vs 121 and 37 750 vs 133 ELISA units, respectively), hACE2 receptor binding inhibition GM titers (GMTs) (222 vs 5 and 136 vs 5, respectively), and neutralizing antibody GMTs (1303 vs 11 and 900 vs 11, respectively). Humoral responses were 30-40% lower in participants ≥65 years or HIV-positive; however, seroconversion rates were high and comparable between the age cohorts, regardless of HIV serostatus.
Conclusions: NVX-CoV2373 elicited robust humoral immune responses against ancestral SARS-CoV-2 virus 2 weeks following the second vaccination in adult PREVENT-19 participants, consistent with previously reported high vaccine efficacy.
Conflict of interest statement
Potential conflicts of interest. GÁ, JN, HD, SC-C, AMG, WW, IC, JSP, GMG, and LMD are or were employees of Novavax, Inc. and may own stock or stock options. KLK and CLG do not report conflicts of interest.
Figures

References
-
- Slaoui M, Hepburn M. Developing safe and effective covid vaccines — Operation Warp Speed’s strategy and approach. N Engl J Med 2020;383:1701–3. - PubMed
-
- World Health Organization. Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. January 12, 2023. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVI.... Accessed 12 March 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
