This is a preprint.
Detection of a geminate photoproduct of bovine cytochrome c oxidase by time-resolved serial femtosecond crystallography
- PMID: 37214971
- PMCID: PMC10197551
- DOI: 10.1101/2023.05.08.539888
Detection of a geminate photoproduct of bovine cytochrome c oxidase by time-resolved serial femtosecond crystallography
Update in
-
Detection of a Geminate Photoproduct of Bovine Cytochrome c Oxidase by Time-Resolved Serial Femtosecond Crystallography.J Am Chem Soc. 2023 Oct 18;145(41):22305-22309. doi: 10.1021/jacs.3c07803. Epub 2023 Sep 11. J Am Chem Soc. 2023. PMID: 37695261 Free PMC article.
Abstract
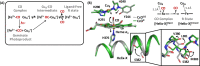
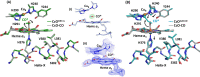
Cytochrome c oxidase (C c O) is a large membrane-bound hemeprotein that catalyzes the reduction of dioxygen to water. Unlike classical dioxygen binding hemeproteins with a heme b group in their active sites, C c O has a unique binuclear center (BNC) comprised of a copper atom (Cu B ) and a heme a 3 iron, where O 2 binds and is reduced to water. CO is a versatile O 2 surrogate in ligand binding and escape reactions. Previous time-resolved spectroscopic studies of the CO complexes of bovine C c O (bC c O) revealed that photolyzing CO from the heme a 3 iron leads to a metastable intermediate (Cu B -CO), where CO is bound to Cu B , before it escapes out of the BNC. Here, with a time-resolved serial femtosecond X-ray crystallography-based pump-probe method, we detected a geminate photoproduct of the bC c O-CO complex, where CO is dissociated from the heme a 3 iron and moved to a temporary binding site midway between the Cu B and the heme a 3 iron, while the locations of the two metal centers and the conformation of the Helix-X, housing the proximal histidine ligand of the heme a 3 iron, remain in the CO complex state. This new structure, combined with other reported structures of bC c O, allows the full definition of the ligand dissociation trajectory, as well as the associated protein dynamics.
Figures

References
-
- Han S., Takahashi S. & Rousseau D. L. Time dependence of the catalytic intermediates in cytochrome c oxidase. The Journal of biological chemistry 275, 1910–1919 (2000). - PubMed
-
- Kitagawa T. & Ogura T. Time-resolved resonance Raman investigation of oxygen reduction mechanism of bovine cytochrome c oxidase. Journal of bioenergetics and biomembranes 30, 71–79 (1998). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
