This is a preprint.
The Regulator FleQ Post-Transcriptionally Regulates the Production of RTX Adhesins by Pseudomonas fluorescens
- PMID: 37214974
- PMCID: PMC10197612
- DOI: 10.1101/2023.05.09.540025
The Regulator FleQ Post-Transcriptionally Regulates the Production of RTX Adhesins by Pseudomonas fluorescens
Update in
-
The regulator FleQ both transcriptionally and post-transcriptionally regulates the level of RTX adhesins of Pseudomonas fluorescens.J Bacteriol. 2023 Sep 26;205(9):e0015223. doi: 10.1128/jb.00152-23. Epub 2023 Sep 1. J Bacteriol. 2023. PMID: 37655913 Free PMC article.
Abstract
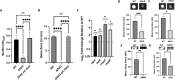
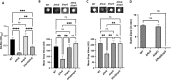
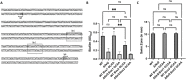
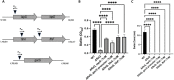
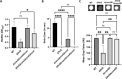
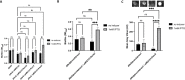
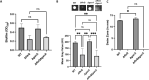
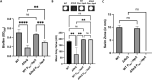
Biofilm formation by the Gram-negative gammaproteobacterium Pseudomonas fluorescens relies on the production of the repeat-in-toxin (RTX) adhesins LapA and MapA in the cytoplasm, secretion of these adhesins through their respective type 1 secretion systems, and retention at the cell surface. Published work has shown that retention of the adhesins occurs via a post-translational mechanism involving the cyclic-di-GMP receptor LapD and the protease LapG. However, little is known about the underlying mechanisms that regulate the production of these adhesins. Here, we demonstrate that the master regulator FleQ modulates biofilm formation by post-transcriptionally regulating the production of LapA and MapA. We find that a Δ fleQ mutant has a biofilm formation defect compared to the WT strain, which is attributed in part to a decrease in LapA and MapA production, despite the Δ fleQ mutant having increased levels of lapA and mapA transcripts compared to the WT strain. Through transposon mutagenesis and subsequent genetic analysis, we found that over-stimulation of the Gac/Rsm pathway partially rescues biofilm formation in the Δ fleQ mutant background. Collectively, these findings provide evidence that FleQ regulates biofilm formation by post-transcriptionally regulating the production of LapA and MapA, and that activation of the Gac/Rsm pathway can enhance biofilm formation by P. fluorescens .
Importance: Biofilm formation is a highly coordinated process that bacteria undergo to colonize a variety of surfaces. For Pseudomonas fluorescens , biofilm formation requires the production and localization of RTX adhesins to the cell surface. To date, little is known about the underlying mechanisms that regulate biofilm formation by P. fluorescens . Here, we identify FleQ as a key regulator of biofilm formation that modulates the production of LapA and MapA through a post-transcriptional mechanism. We provide further evidence implicating activation of the Gac/Rsm system in FleQ-dependent regulation of biofilm formation. Together, our findings uncover evidence for a mechanism of post-transcriptional regulation of the LapA/MapA adhesins.
Figures
References
-
- Paulsen IT, Press CM, Ravel J, Kobayashi DY, Myers GSA, Mavrodi DV, DeBoy RT, Seshadri R, Ren Q, Madupu R, Dodson RJ, Durkin AS, Brinkac LM, Daugherty SC, Sullivan SA, Rosovitz MJ, Gwinn ML, Zhou L, Schneider DJ, Cartinhour SW, Nelson WC, Weidman J, Watkins K, Tran K, Khouri H, Pierson EA, Pierson LS, Thomashow LS, Loper JE. 2005. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat Biotechnol 23:873–878. - PMC - PubMed
-
- Howell CR. 1979. Control of Rhizoctonia solani on cotton seedlings with Pseudomonas fluorescens and with an antibiotic produced by the bacterium. J Phytopathol 69:480.
-
- Keel C. 1992. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. MPMI 5:4.
-
- Haas D, Défago G. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319. - PubMed
-
- Khabbaz RF, Arnow PM, Highsmith AK, Herwaldt LA, Chou T, Jarvis WR, Lerche NW, Allen JR. 1984. Pseudomonas fluorescens bacteremia from blood transfusion. Am J Med 76:62–68. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
