This is a preprint.
POT1 recruits and regulates CST-Polα/Primase at human telomeres
- PMID: 37215005
- PMCID: PMC10197580
- DOI: 10.1101/2023.05.08.539880
POT1 recruits and regulates CST-Polα/Primase at human telomeres
Update in
-
POT1 recruits and regulates CST-Polα/primase at human telomeres.Cell. 2024 Jul 11;187(14):3638-3651.e18. doi: 10.1016/j.cell.2024.05.002. Epub 2024 Jun 4. Cell. 2024. PMID: 38838667 Free PMC article.
Abstract
Telomere maintenance requires extension of the G-rich telomeric repeat strand by telomerase and fill-in synthesis of the C-rich strand by Polα/Primase. Telomeric Polα/Primase is bound to Ctc1-Stn1-Ten1 (CST), a single-stranded DNA-binding complex. Like mutations in telomerase, mutations affecting CST-Polα/Primase result in pathological telomere shortening and cause a telomere biology disorder, Coats plus (CP). We determined cryogenic electron microscopy structures of human CST bound to the shelterin heterodimer POT1/TPP1 that reveal how CST is recruited to telomeres by POT1. Phosphorylation of POT1 is required for CST recruitment, and the complex is formed through conserved interactions involving several residues mutated in CP. Our structural and biochemical data suggest that phosphorylated POT1 holds CST-Polα/Primase in an inactive auto-inhibited state until telomerase has extended the telomere ends. We propose that dephosphorylation of POT1 releases CST-Polα/Primase into an active state that completes telomere replication through fill-in synthesis.
Keywords: CST; DNA replication; DNA-protein complex; POT1; Polymerase α/Primase; cryo-EM; phospho-regulation; shelterin; telomere.
Conflict of interest statement
Competing interests Titia de Lange is a member of the SAB of Calico Life Sciences LLC, San Francisco. The other authors have no conflicts to declare.
Figures

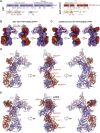
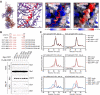


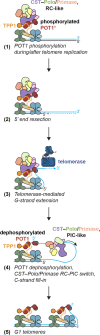
References
-
- de Lange T. Shelterin-Mediated Telomere Protection. Annu Rev Genet 52, 223–247 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
