This is a preprint.
Elevated nuclear TDP-43 induces constitutive exon skipping
- PMID: 37215013
- PMCID: PMC10197708
- DOI: 10.1101/2023.05.11.540291
Elevated nuclear TDP-43 induces constitutive exon skipping
Update in
-
Elevated nuclear TDP-43 induces constitutive exon skipping.Mol Neurodegener. 2024 Jun 9;19(1):45. doi: 10.1186/s13024-024-00732-w. Mol Neurodegener. 2024. PMID: 38853250 Free PMC article.
Abstract
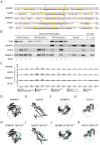
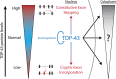
Cytoplasmic inclusions and loss of nuclear TDP-43 are key pathological features found in several neurodegenerative disorders, suggesting both gain- and loss-of-function mechanisms of disease. To study gain-of-function, TDP-43 overexpression has been used to generate in vitro and in vivo model systems. Our study shows that excessive levels of nuclear TDP-43 protein lead to constitutive exon skipping that is largely species-specific. Furthermore, while aberrant exon skipping is detected in some human brains, it is not correlated with disease, unlike the incorporation of cryptic exons that occurs after loss of TDP-43. Our findings emphasize the need for caution in interpreting TDP-43 overexpression data, and stress the importance of controlling for exon skipping when generating models of TDP-43 proteinopathy. Understanding the subtle aspects of TDP-43 toxicity within different subcellular locations is essential for the development of therapies targeting neurodegenerative disease.
Figures



Similar articles
-
Elevated nuclear TDP-43 induces constitutive exon skipping.Mol Neurodegener. 2024 Jun 9;19(1):45. doi: 10.1186/s13024-024-00732-w. Mol Neurodegener. 2024. PMID: 38853250 Free PMC article.
-
Cryptic exon incorporation occurs in Alzheimer's brain lacking TDP-43 inclusion but exhibiting nuclear clearance of TDP-43.Acta Neuropathol. 2017 Jun;133(6):923-931. doi: 10.1007/s00401-017-1701-2. Epub 2017 Mar 22. Acta Neuropathol. 2017. PMID: 28332094 Free PMC article.
-
Optogenetic TDP-43 nucleation induces persistent insoluble species and progressive motor dysfunction in vivo.Neurobiol Dis. 2020 Dec;146:105078. doi: 10.1016/j.nbd.2020.105078. Epub 2020 Sep 12. Neurobiol Dis. 2020. PMID: 32927062 Free PMC article.
-
Emerging Therapies and Novel Targets for TDP-43 Proteinopathy in ALS/FTD.Neurotherapeutics. 2022 Jul;19(4):1061-1084. doi: 10.1007/s13311-022-01260-5. Epub 2022 Jul 5. Neurotherapeutics. 2022. PMID: 35790708 Free PMC article. Review.
-
TDP-43 pathology: From noxious assembly to therapeutic removal.Prog Neurobiol. 2022 Apr;211:102229. doi: 10.1016/j.pneurobio.2022.102229. Epub 2022 Jan 29. Prog Neurobiol. 2022. PMID: 35101542 Review.
Cited by
-
A Slower-Progressing TDP-43 rNLS8 Mouse Model for ALS: Implications for Preclinical and Mechanistic Studies.Neuromolecular Med. 2025 Aug 18;27(1):59. doi: 10.1007/s12017-025-08871-z. Neuromolecular Med. 2025. PMID: 40824324 Free PMC article.
References
-
- Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., Feiden W., Kretzschmar H. A., Trojanowski J. Q., Lee V. M.-Y., Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 314, 130–133 (2006). - PubMed
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T., TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006). - PubMed
-
- Nelson P. T., Dickson D. W., Trojanowski J. Q., Jack C. R., Boyle P. A., Arfanakis K., Rademakers R., Alafuzoff I., Attems J., Brayne C., Coyle-Gilchrist I. T. S., Chui H. C., Fardo D. W., Flanagan M. E., Halliday G., Hokkanen S. R. K., Hunter S., Jicha G. A., Katsumata Y., Kawas C. H., Keene C. D., Kovacs G. G., Kukull W. A., Levey A. I., Makkinejad N., Montine T. J., Murayama S., Murray M. E., Nag S., Rissman R. A., Seeley W. W., Sperling R. A., White C. L. 3rd, Yu L., Schneider J. A., Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 142, 1503–1527 (2019). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
