Cartilage regeneration in zebrafish depends on Nrg1/ErbB signaling pathway
- PMID: 37215080
- PMCID: PMC10192884
- DOI: 10.3389/fcell.2023.1123299
Cartilage regeneration in zebrafish depends on Nrg1/ErbB signaling pathway
Abstract
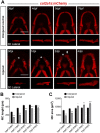
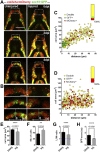
Objective: Cartilage, as the majority of adult mammalian tissues, has limited regeneration capacity. Cartilage degradation consecutive to joint injury or aging then leads to irreversible joint damage and diseases. In contrast, several vertebrate species such as the zebrafish have the remarkable capacity to spontaneously regenerate skeletal structures after severe injuries. The objective of our study was to test the regenerative capacity of Meckel's cartilage (MC) upon mechanical injury in zebrafish and to identify the mechanisms underlying this process. Methods and Results: Cartilage regenerative capacity in zebrafish larvae was investigated after mechanical injuries of the lower jaw MC in TgBAC(col2a1a:mCherry), to visualize the loss and recovery of cartilage. Confocal analysis revealed the formation of new chondrocytes and complete regeneration of MC at 14 days post-injury (dpi) via chondrocyte cell cycle re-entry and proliferation of pre-existing MC chondrocytes near the wound. Through expression analyses, we showed an increase of nrg1 expression in the regenerating lower jaw, which also expresses Nrg1 receptors, ErbB3 and ErbB2. Pharmacological inhibition of the ErbB pathway and specific knockdown of Nrg1 affected MC regeneration indicating the pivotal role of this pathway for cartilage regeneration. Finally, addition of exogenous NRG1 in an in vitro model of osteoarthritic (OA)-like chondrocytes induced by IL1β suggests that Nrg1/ErbB pathway is functional in mammalian chondrocytes and alleviates the increased expression of catabolic markers characteristic of OA-like chondrocytes. Conclusion: Our results show that the Nrg1/ErbB pathway is required for spontaneous cartilage regeneration in zebrafish and is of interest to design new therapeutic approaches to promote cartilage regeneration in mammals.
Keywords: cartilage; chrondrocytes; neuregulin 1; osteoarthritis; regeneration.
Copyright © 2023 Sapède, Bahraoui, Abou Nassif, Barthelaix, Mathieu, Jorgensen and Djouad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Regeneration of Jaw Joint Cartilage in Adult Zebrafish.Front Cell Dev Biol. 2022 Jan 20;9:777787. doi: 10.3389/fcell.2021.777787. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35127702 Free PMC article.
-
Tissue regeneration during lower jaw restoration in zebrafish shows some features of epimorphic regeneration.Dev Growth Differ. 2019 Sep;61(7-8):419-430. doi: 10.1111/dgd.12625. Epub 2019 Aug 29. Dev Growth Differ. 2019. PMID: 31468519
-
Ihha induces hybrid cartilage-bone cells during zebrafish jawbone regeneration.Development. 2016 Jun 15;143(12):2066-76. doi: 10.1242/dev.131292. Epub 2016 Apr 27. Development. 2016. PMID: 27122168 Free PMC article.
-
Cartilage regeneration and ageing: Targeting cellular plasticity in osteoarthritis.Ageing Res Rev. 2018 Mar;42:56-71. doi: 10.1016/j.arr.2017.12.006. Epub 2017 Dec 16. Ageing Res Rev. 2018. PMID: 29258883 Review.
-
A Comprehensive Review of Stem Cells for Cartilage Regeneration in Osteoarthritis.Adv Exp Med Biol. 2018;1089:23-36. doi: 10.1007/5584_2018_205. Adv Exp Med Biol. 2018. PMID: 29725971 Review.
Cited by
-
Characterization of a chemically induced osteoarthritis model in zebrafish.Sci Rep. 2025 Jan 31;15(1):3905. doi: 10.1038/s41598-025-88125-x. Sci Rep. 2025. PMID: 39890962 Free PMC article.
-
Histone acetylation facilitates multidirectional pulp repair through Neuregulin-1 mobilization.Stem Cells Transl Med. 2025 Jun 25;14(7):szaf022. doi: 10.1093/stcltm/szaf022. Stem Cells Transl Med. 2025. PMID: 40580029 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

