Mechanobiology and survival strategies of circulating tumor cells: a process towards the invasive and metastatic phenotype
- PMID: 37215087
- PMCID: PMC10196185
- DOI: 10.3389/fcell.2023.1188499
Mechanobiology and survival strategies of circulating tumor cells: a process towards the invasive and metastatic phenotype
Abstract
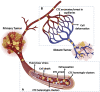
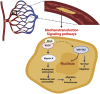
Metastatic progression is the deadliest feature of cancer. Cancer cell growth, invasion, intravasation, circulation, arrest/adhesion and extravasation require specific mechanical properties to allow cell survival and the completion of the metastatic cascade. Circulating tumor cells (CTCs) come into contact with the capillary bed during extravasation/intravasation at the beginning of the metastatic cascade. However, CTC mechanobiology and survival strategies in the bloodstream, and specifically in the microcirculation, are not well known. A fraction of CTCs can extravasate and colonize distant areas despite the biomechanical constriction forces that are exerted by the microcirculation and that strongly decrease tumor cell survival. Furthermore, accumulating evidence shows that several CTC adaptations, via molecular factors and interactions with blood components (e.g., immune cells and platelets inside capillaries), may promote metastasis formation. To better understand CTC journey in the microcirculation as part of the metastatic cascade, we reviewed how CTC mechanobiology and interaction with other cell types in the bloodstream help them to survive the harsh conditions in the circulatory system and to metastasize in distant organs.
Keywords: cancer; circulating tumor cells; mechanobiology; metastasis; survival.
Copyright © 2023 Kurma and Alix-Panabières.
Conflict of interest statement
CA-P is one of the patent holders (US Patent Number 16093934) for detecting and/or characterizing circulating tumor cells. She received an honorarium from Menarini. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Squeezing through the microcirculation: survival adaptations of circulating tumour cells to seed metastasis.Br J Cancer. 2021 Jan;124(1):58-65. doi: 10.1038/s41416-020-01176-x. Epub 2020 Dec 1. Br J Cancer. 2021. PMID: 33257836 Free PMC article. Review.
-
Microenvironmental Influences on Metastasis Suppressor Expression and Function during a Metastatic Cell's Journey.Cancer Microenviron. 2014 Dec;7(3):117-31. doi: 10.1007/s12307-014-0148-4. Epub 2014 Jun 18. Cancer Microenviron. 2014. PMID: 24938990 Free PMC article.
-
In vitro cross-talk between metastasis-competent circulating tumor cells and platelets in colon cancer: a malicious association during the harsh journey in the blood.Front Cell Dev Biol. 2023 Aug 2;11:1209846. doi: 10.3389/fcell.2023.1209846. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37601099 Free PMC article.
-
S-Nitrosocaptopril prevents cancer metastasis in vivo by creating the hostile bloodstream microenvironment against circulating tumor cells.Pharmacol Res. 2019 Jan;139:535-549. doi: 10.1016/j.phrs.2018.10.020. Epub 2018 Oct 23. Pharmacol Res. 2019. PMID: 30366102
-
Mechanical deformation and death of circulating tumor cells in the bloodstream.Cancer Metastasis Rev. 2024 Dec;43(4):1489-1510. doi: 10.1007/s10555-024-10198-3. Epub 2024 Jul 9. Cancer Metastasis Rev. 2024. PMID: 38980581 Free PMC article. Review.
Cited by
-
An innovative cellular medicine approach via the utilization of novel nanotechnology-based biomechatronic platforms as a label-free biomarker for early melanoma diagnosis.Sci Rep. 2024 Dec 3;14(1):30107. doi: 10.1038/s41598-024-79154-z. Sci Rep. 2024. PMID: 39627312 Free PMC article.
-
Surfaceome: a new era in the discovery of immune evasion mechanisms of circulating tumor cells.Mol Oncol. 2025 Jul;19(7):1979-1997. doi: 10.1002/1878-0261.13665. Epub 2024 May 22. Mol Oncol. 2025. PMID: 38775116 Free PMC article. Review.
-
Epithelial and mesenchymal phenotypes determine the dynamics of circulating breast tumor cells in microfluidic capillaries under chemotherapy-induced stress.Biomicrofluidics. 2024 Apr 5;18(2):024106. doi: 10.1063/5.0188861. eCollection 2024 Mar. Biomicrofluidics. 2024. PMID: 38585003 Free PMC article.
-
Phenotypic Transitions the Processes Involved in Regulation of Growth and Proangiogenic Properties of Stem Cells, Cancer Stem Cells and Circulating Tumor Cells.Stem Cell Rev Rep. 2024 May;20(4):967-979. doi: 10.1007/s12015-024-10691-w. Epub 2024 Feb 19. Stem Cell Rev Rep. 2024. PMID: 38372877 Free PMC article. Review.
-
Adipocyte-derived factors induce adherent to suspension transition in breast and pancreatic cancer cells through lipid metabolic alteration.Sci Rep. 2025 Aug 20;15(1):30552. doi: 10.1038/s41598-025-13309-4. Sci Rep. 2025. PMID: 40836054 Free PMC article.
References
-
- Alix-Panabières C., Pantel K. (2021). Liquid biopsy: From discovery to clinical application. Cancer Discov. 11 (4), 858–873. 10.1158/2159-8290.cd-20-1311 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

