Remission of social behavior impairment by oral administration of a precursor of NAD in CD157, but not in CD38, knockout mice
- PMID: 37215105
- PMCID: PMC10192747
- DOI: 10.3389/fimmu.2023.1166609
Remission of social behavior impairment by oral administration of a precursor of NAD in CD157, but not in CD38, knockout mice
Abstract
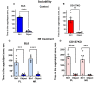
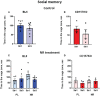
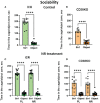
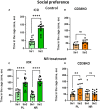
Nicotinamide adenine dinucleotide (NAD) is a substrate of adenosine diphosphate (ADP)-ribosyl cyclase and is catalyzed to cyclic ADP-ribose (cADPR) by CD38 and/or CD157. cADPR, a Ca2+ mobilizing second messenger, is critical in releasing oxytocin from the hypothalamus into the brain. Although NAD precursors effectively play a role in neurodegenerative disorders, muscular dystrophy, and senescence, the beneficial effects of elevating NAD by NAD precursor supplementation on brain function, especially social interaction, and whether CD38 is required in this response, has not been intensely studied. Here, we report that oral gavage administration of nicotinamide riboside, a perspective NAD precursor with high bioavailability, for 12 days did not show any suppressive or increasing effects on sociability (mouse's interest in social targets compared to non-social targets) in both CD157KO and CD38KO male mice models in a three-chamber test. CD157KO and CD38KO mice displayed no social preference (that is, more interest towards a novel mouse than a familiar one) behavior. This defect was rescued after oral gavage administration of nicotinamide riboside for 12 days in CD157KO mice, but not in CD38KO mice. Social memory was not observed in CD157KO and CD38KO mice; subsequently, nicotinamide riboside administration had no effect on social memory. Together with the results that nicotinamide riboside had essentially no or little effect on body weight during treatment in CD157KO mice, nicotinamide riboside is less harmful and has beneficial effect on defects in recovery from social behavioral, for which CD38 is required in mice.
Keywords: CD157; CD38; NAD; neuromodulator; nicotinamide riboside; social behavior.
Copyright © 2023 Gerasimenko and Higashida.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Nicotinamide riboside supplementation corrects deficits in oxytocin, sociability and anxiety of CD157 mutants in a mouse model of autism spectrum disorder.Sci Rep. 2020 Jun 22;10(1):10035. doi: 10.1038/s41598-019-57236-7. Sci Rep. 2020. PMID: 32572044 Free PMC article.
-
Distinct physical condition and social behavior phenotypes of CD157 and CD38 knockout mice during aging.PLoS One. 2020 Dec 16;15(12):e0244022. doi: 10.1371/journal.pone.0244022. eCollection 2020. PLoS One. 2020. PMID: 33326496 Free PMC article.
-
CD38, CD157, and RAGE as Molecular Determinants for Social Behavior.Cells. 2019 Dec 25;9(1):62. doi: 10.3390/cells9010062. Cells. 2019. PMID: 31881755 Free PMC article. Review.
-
Identification of a major enzyme for the synthesis and hydrolysis of cyclic ADP-ribose in amphibian cells and evolutional conservation of the enzyme from human to invertebrate.Mol Cell Biochem. 2012 Jul;366(1-2):69-80. doi: 10.1007/s11010-012-1284-0. Epub 2012 Mar 16. Mol Cell Biochem. 2012. PMID: 22422046
-
The role of dietary niacin intake and the adenosine-5'-diphosphate-ribosyl cyclase enzyme CD38 in spatial learning ability: is cyclic adenosine diphosphate ribose the link between diet and behaviour?Nutr Res Rev. 2008 Jun;21(1):42-55. doi: 10.1017/S0954422408945182. Nutr Res Rev. 2008. PMID: 19079853 Review.
Cited by
-
Nicotinamide Riboside, a Promising Vitamin B3 Derivative for Healthy Aging and Longevity: Current Research and Perspectives.Molecules. 2023 Aug 15;28(16):6078. doi: 10.3390/molecules28166078. Molecules. 2023. PMID: 37630330 Free PMC article. Review.
-
Pathobiochemistry of Aging and Neurodegeneration: Deregulation of NAD+ Metabolism in Brain Cells.Biomolecules. 2024 Dec 6;14(12):1556. doi: 10.3390/biom14121556. Biomolecules. 2024. PMID: 39766263 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

