Open drug discovery in Alzheimer's disease
- PMID: 37215505
- PMCID: PMC10192886
- DOI: 10.1002/trc2.12394
Open drug discovery in Alzheimer's disease
Abstract
Alzheimer's disease (AD) drug discovery has focused on a set of highly studied therapeutic hypotheses, with limited success. The heterogeneous nature of AD processes suggests that a more diverse, systems-integrated strategy may identify new therapeutic hypotheses. Although many target hypotheses have arisen from systems-level modeling of human disease, in practice and for many reasons, it has proven challenging to translate them into drug discovery pipelines. First, many hypotheses implicate protein targets and/or biological mechanisms that are under-studied, meaning there is a paucity of evidence to inform experimental strategies as well as high-quality reagents to perform them. Second, systems-level targets are predicted to act in concert, requiring adaptations in how we characterize new drug targets. Here we posit that the development and open distribution of high-quality experimental reagents and informatic outputs-termed target enabling packages (TEPs)-will catalyze rapid evaluation of emerging systems-integrated targets in AD by enabling parallel, independent, and unencumbered research.
Keywords: Alzheimer's disease; TEPs; drug development; drug targets; genomics; proteomics; systems biology.
© 2023 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting Information.
Figures
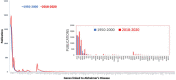
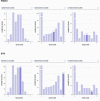
References
-
- WHO . Global action plan on the public health response to dementia. Geneva: WHO. 2017;2017‐2025.
-
- Liu KY, Howard R. Can we learn lessons from the FDA's approval of aducanumab? Nat Rev Neurol. 2021;17:715‐722. - PubMed
-
- Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163‐165. - PubMed
-
- GWAS Catalog. https://www.ebi.ac.uk/gwas/efotraits/EFO_0000249
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
