MOF-derived bimetallic nanozyme to catalyze ROS scavenging for protection of myocardial injury
- PMID: 37215581
- PMCID: PMC10196836
- DOI: 10.7150/thno.83543
MOF-derived bimetallic nanozyme to catalyze ROS scavenging for protection of myocardial injury
Abstract
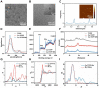
Rationale: Myocardial injury triggers intense oxidative stress, inflammatory response, and cytokine release, which are essential for myocardial repair and remodeling. Excess reactive oxygen species (ROS) scavenging and inflammation elimination have long been considered to reverse myocardial injuries. However, the efficacy of traditional treatments (antioxidant, anti-inflammatory drugs and natural enzymes) is still poor due to their intrinsic defects such as unfavorable pharmacokinetics and bioavailability, low biological stability, and potential side effects. Nanozyme represents a candidate to effectively modulate redox homeostasis for the treatment of ROS related inflammation diseases. Methods: We develop an integrated bimetallic nanozyme derived from metal-organic framework (MOF) to eliminate ROS and alleviate inflammation. The bimetallic nanozyme (Cu-TCPP-Mn) is synthesized by embedding manganese and copper into the porphyrin followed by sonication, which could mimic the cascade activities of superoxide dismutase (SOD) and catalase (CAT) to transform oxygen radicals to hydrogen peroxide, followed by the catalysis of hydrogen peroxide into oxygen and water. Enzyme kinetic analysis and oxygen-production velocities analysis were performed to evaluate the enzymatic activities of Cu-TCPP-Mn. We also established myocardial infarction (MI) and myocardial ischemia-reperfusion (I/R) injury animal models to verify the ROS scavenging and anti-inflammation effect of Cu-TCPP-Mn. Results: As demonstrated by kinetic analysis and oxygen-production velocities analysis, Cu-TCPP-Mn nanozyme possesses good performance in both SOD- and CAT-like activities to achieve synergistic ROS scavenging effect and provide protection for myocardial injury. In both MI and I/R injury animal models, this bimetallic nanozyme represents a promising and reliable technology to protect the heart tissue from oxidative stress and inflammation-induced injury, and enables the myocardial function to recover from otherwise severe damage. Conclusions: This research provides a facile and applicable method to develop a bimetallic MOF nanozyme, which represents a promising alternative to the treatment of myocardial injuries.
Keywords: metal-organic framework; myocardial injury; nanomedicine; nanozyme; reactive oxygen species.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Zhang Z, Dalan R, Hu Z, Wang JW, Chew N, Poh KK. et al. Reactive oxygen species scavenging nanomedicine for the treatment of ischemic heart disease. Adv Mater. 2022;34(35):e2022169. - PubMed
-
- Yao J, Huang K, Zhu D, Chen T, Jiang Y, Zhang J. et al. A minimally invasive exosome spray repairs heart after myocardial infarction. ACS Nano. 2021;15(7):11099–111. - PubMed
-
- Li HL, Zhuo ML, Wang D, Wang AB, Cai H, Sun LH. et al. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation. 2007;115(14):1885–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

