Modeling of pulmonary deposition of agents of open and fixed dose triple combination therapies through two different low-resistance inhalers in COPD: a pilot study
- PMID: 37215734
- PMCID: PMC10196142
- DOI: 10.3389/fmed.2023.1065072
Modeling of pulmonary deposition of agents of open and fixed dose triple combination therapies through two different low-resistance inhalers in COPD: a pilot study
Abstract
Introduction: Inhalation therapy is a cornerstone of treating patients with chronic obstructive pulmonary disease (COPD). Inhaler devices might influence the effectiveness of inhalation therapy. We aimed to model and compare the deposition of acting agents of an open and a fixed dose combination (FDC) triple therapy and examine their repeatability.
Methods: We recruited control subjects (Controls, n = 17) and patients with stable COPD (S-COPD, n = 13) and those during an acute exacerbation (AE-COPD, n = 12). Standard spirometry was followed by through-device inhalation maneuvers using a pressurized metered dose inhaler (pMDI) and a soft mist inhaler (SMI) to calculate deposition of fixed dose and open triple combination therapies by numerical modeling. Through-device inspiratory vital capacity (IVCd) and peak inspiratory flow (PIFd), as well as inhalation time (tin) and breath hold time (tbh) were used to calculate pulmonary (PD) and extrathoracic deposition (ETD) values. Deposition was calculated from two different inhalation maneuvers.
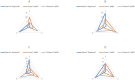
Results: There was no difference in forced expiratory volume in 1 s (FEV1) between patients (S-COPD: 42 ± 5% vs. AE-COPD: 35 ± 5% predicted). Spiriva® Respimat® showed significantly higher PD and lower ETD values in all COPD patients and Controls compared with the two pMDIs. For Foster® pMDI and Trimbow® pMDI similar PD were observed in Controls, while ETD between Controls and AE-COPD patients did significantly differ. There was no difference between COPD groups regarding the repeatability of calculated deposition values. Ranking the different inhalers by differences between the two deposition values calculated from separate maneuvers, Respimat® produced the smallest inter-measurement differences for PD.
Discussion: Our study is the first to model and compare PD using pMDIs and an SMI as triple combination in COPD. In conclusion, switching from FDC to open triple therapy in cases when adherence to devices is maintanined may contribute to better therapeutic effectiveness in individual cases using low resistance inhalers.
Keywords: COPD; deposition; fixed dose triple therapy; modeling; open triple therapy; repeatability.
Copyright © 2023 Erdelyi, Lazar, Farkas, Furi, Nagy and Müller.
Conflict of interest statement
VM received consultation fees from Astra Zeneca, Boehringer Ingelheim, Chiesi, Berlin Chemie Menarini, Orion Pharma, Novartis, GSK, Teva. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- GOLD , Global Strategy for Diagnosis, Management and Prevention of COPD. Available at: www.gold.copd.org, (2022).
-
- Asthma G.I.F., Global Strategy for Asthma Management and Prevention. Available at: www.ginasthma.org, (2022).
-
- Bosnic-Anticevich S, Chrystyn H, Costello R, Dolovich MB, Fletcher M, Lavorini F, et al. . The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. (2017) 12:59–71. doi: 10.2147/COPD.S117196, PMID: - DOI - PMC - PubMed
-
- Usmani O, Roche N, Wahab E, Israel S, Jenkins M, Trivedi R, et al. . A scintigraphy study of budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler in patients with moderate-to-very severe chronic obstructive pulmonary disease. Respir Res. (2021) 22:261. doi: 10.1186/s12931-021-01813-w, PMID: - DOI - PMC - PubMed
-
- Wei X, Hindle M, Kaviratna A, Huynh BK, Delvadia RR, Sandell D, et al. . In vitro tests for aerosol deposition. VI: realistic testing with different mouth-throat models and in vitro-in vivo correlations for a dry powder inhaler, metered dose inhaler, and soft mist inhaler. J Aerosol Med Pulm Drug Deliv. (2018) 31:358–71. doi: 10.1089/jamp.2018.1454, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

