Brain-derived extracellular vesicles promote bone-fat imbalance in Alzheimer's disease
- PMID: 37215980
- PMCID: PMC10197897
- DOI: 10.7150/ijbs.79461
Brain-derived extracellular vesicles promote bone-fat imbalance in Alzheimer's disease
Abstract
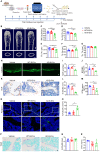
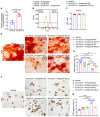
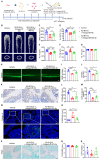
Inadequate osteogenesis and excessive adipogenesis of bone marrow mesenchymal stem cells (BMSCs) are key factors in the pathogenesis of osteoporosis. Patients with Alzheimer's disease (AD) have a higher incidence of osteoporosis than healthy adults, but the underlying mechanism is not clear. Here, we show that brain-derived extracellular vesicles (EVs) from adult AD or wild-type mice can cross the blood-brain barrier to reach the distal bone tissue, while only AD brain-derived EVs (AD-B-EVs) significantly promote the shift of the BMSC differentiation fate from osteogenesis to adipogenesis and induce a bone-fat imbalance. MiR-483-5p is highly enriched in AD-B-EVs, brain tissues from AD mice, and plasma-derived EVs from AD patients. This miRNA mediates the anti-osteogenic, pro-adipogenic, and pro-osteoporotic effects of AD-B-EVs by inhibiting Igf2. This study identifies the role of B-EVs as a promoter of osteoporosis in AD by transferring miR-483-5p.
Keywords: Alzheimer's disease; adipogenesis; extracellular vesicles; miR-483-5p; osteoporosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
BMSC-derived extracellular vesicles promoted osteogenesis via Axin2 inhibition by delivering MiR-16-5p.Int Immunopharmacol. 2023 Jul;120:110319. doi: 10.1016/j.intimp.2023.110319. Epub 2023 May 20. Int Immunopharmacol. 2023. PMID: 37216799
-
Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism.Theranostics. 2020 Jan 16;10(5):2293-2308. doi: 10.7150/thno.39238. eCollection 2020. Theranostics. 2020. PMID: 32089743 Free PMC article.
-
MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence.Tissue Eng Part A. 2017 Nov;23(21-22):1231-1240. doi: 10.1089/ten.TEA.2016.0525. Epub 2017 Apr 28. Tissue Eng Part A. 2017. PMID: 28363268 Free PMC article.
-
MicroRNAs in Extracellular Vesicles of Alzheimer's Disease.Cells. 2023 May 13;12(10):1378. doi: 10.3390/cells12101378. Cells. 2023. PMID: 37408212 Free PMC article. Review.
-
Crosstalk between bone and brain in Alzheimer's disease: Mechanisms, applications, and perspectives.Alzheimers Dement. 2024 Aug;20(8):5720-5739. doi: 10.1002/alz.13864. Epub 2024 Jun 2. Alzheimers Dement. 2024. PMID: 38824621 Free PMC article. Review.
Cited by
-
Extracellular vesicle therapy in neurological disorders.J Biomed Sci. 2024 Aug 25;31(1):85. doi: 10.1186/s12929-024-01075-w. J Biomed Sci. 2024. PMID: 39183263 Free PMC article. Review.
-
Microbiota-bone axis in ageing-related bone diseases.Front Endocrinol (Lausanne). 2024 Jul 15;15:1414350. doi: 10.3389/fendo.2024.1414350. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39076510 Free PMC article. Review.
-
Young osteocyte-derived extracellular vesicles facilitate osteogenesis by transferring tropomyosin-1.J Nanobiotechnology. 2024 Apr 25;22(1):208. doi: 10.1186/s12951-024-02367-x. J Nanobiotechnology. 2024. PMID: 38664789 Free PMC article.
-
Osteoporotic osseointegration: therapeutic hallmarks and engineering strategies.Theranostics. 2024 Jun 17;14(10):3859-3899. doi: 10.7150/thno.96516. eCollection 2024. Theranostics. 2024. PMID: 38994021 Free PMC article. Review.
-
Peripheral extracellular vesicles in neurodegeneration: pathogenic influencers and therapeutic vehicles.J Nanobiotechnology. 2024 Apr 12;22(1):170. doi: 10.1186/s12951-024-02428-1. J Nanobiotechnology. 2024. PMID: 38610012 Free PMC article. Review.
References
-
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. - PubMed
-
- Pu Z, Tang X, Fei Y, Hou Q, Lin Y, Zha X. Bone metabolic biomarkers and bone mineral density in male patients with early-stage Alzheimer's disease. Eur Geriatr Med. 2020;11:403–8. - PubMed
-
- Liu D, Zhou H, Tao Y, Tan J, Chen L, Huang H. et al. Alzheimer's Disease is Associated with Increased Risk of Osteoporosis: The Chongqing Aging Study. Current Alzheimer research. 2016;13:1165–72. - PubMed
-
- Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet (London, England) 2019;393:364–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

