Treating oligodendroglioma - An analysis of a homogeneous 1p/19q-codeleted and isocitrate dehydrogenase-mutant patient cohort
- PMID: 37216045
- PMCID: PMC10192391
- DOI: 10.1016/j.ctro.2023.100626
Treating oligodendroglioma - An analysis of a homogeneous 1p/19q-codeleted and isocitrate dehydrogenase-mutant patient cohort
Abstract
Background: Oligodendrogliomas (ODG) are rare, diffusely infiltrating brain tumors, defined by their 1p/19q-codeletion and isocitrate dehydrogenase (IDH) mutation. Herein, we analyze the influence of various tumor and patient characteristics on progression-free survival (PFS) and overall survival (OS) in a homogeneous patient cohort.
Material and methods: Patients treated for a 1p/19q-codeleted and IDH-mutant ODG were evaluated. The patient and tumor characteristics were analyzed for their influence on PFS and OS.
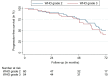
Results: One-hundred-fourteen patients met the inclusion criteria. The median clinical and radiographic follow-up periods were 68.6 and 69.8 months. The median PFS and OS were 66.9 and 236.0 months, respectively. The 2-, 4- and 6-year PFS rates were 89.5%, 76.3%, and 46.0%. The 2-, 4- and 6-year OS rates were 99.0%, 97.9%, and 96.2%. For WHO grade 2 ODG, extent of resection (p = 0.01, hazard ratio (HR) 0.01; p = 0.02, HR 0.02), radiotherapy (p = 0.01, HR < 0.01) and chemotherapy (p = 0.01, HR < 0.01) were associated with a prolonged PFS. For WHO grade 3 ODG, only a combined radiochemotherapy (RCT) lowered the risk of progression in the multivariable analysis (p = 0.02, HR 0.09). Most RCT patients received temozolomide (TMZ) instead of procarbazine, lomustine, and vincristine.
Conclusion: Whereas previous studies often comprise tumors with IDH wild type status and without 1p/19q-codeletion, this homogeneous ODG cohort, as defined by the current WHO classification, demonstrated PFS benefits for various therapies, especially concerning RCT. While this is generally in accordance with comparable studies, more prospective work on homogeneous patient cohorts is required to refine treatment guidelines and to determine the role of TMZ in ODG.
Keywords: IDH mutation; Oligodendroglioma; PCV; Radiochemotherapy; Temozolomide.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
-
- Wick W., Hartmann C., Engel C., Stoffels M., Felsberg J., Stockhammer F., et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. - PubMed
-
- van den Bent M.J., Brandes A.A., Taphoorn M.J.B., Kros J.M., Kouwenhoven M.C.M., Delattre J.-Y., et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous