Optimization of Orally Bioavailable Antileishmanial 2,4,5-Trisubstituted Benzamides
- PMID: 37216489
- PMCID: PMC10259451
- DOI: 10.1021/acs.jmedchem.3c00056
Optimization of Orally Bioavailable Antileishmanial 2,4,5-Trisubstituted Benzamides
Abstract
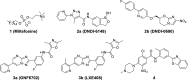
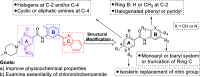
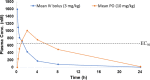
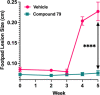
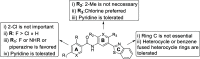
Leishmaniasis, a neglected tropical disease caused by Leishmania species parasites, annually affects over 1 million individuals worldwide. Treatment options for leishmaniasis are limited due to high cost, severe adverse effects, poor efficacy, difficulty of use, and emerging drug resistance to all approved therapies. We discovered 2,4,5-trisubstituted benzamides (4) that possess potent antileishmanial activity but poor aqueous solubility. Herein, we disclose our optimization of the physicochemical and metabolic properties of 2,4,5-trisubstituted benzamide that retains potency. Extensive structure-activity and structure-property relationship studies allowed selection of early leads with suitable potency, microsomal stability, and improved solubility for progression. Early lead 79 exhibited an 80% oral bioavailability and potently blocked proliferation of Leishmania in murine models. These benzamide early leads are suitable for development as orally available antileishmanial drugs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Centers for Disease Control and Prevention , Parasites-Leishmaniasis, https://www.cdc.gov/parasites/leishmaniasis/(accessed Aug 19, 2022).
-
- Nagle A. S.; Khare S.; Kumar A. B.; Supek F.; Buchynskyy A.; Mathison C. J.; Chennamaneni N. K.; Pendem N.; Buckner F. S.; Gelb M. H.; Molteni V. Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem. Rev. 2014, 114, 11305–11347. 10.1021/cr500365f. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

