Dimer-assisted mechanism of (un)saturated fatty acid decarboxylation for alkene production
- PMID: 37216508
- PMCID: PMC10235961
- DOI: 10.1073/pnas.2221483120
Dimer-assisted mechanism of (un)saturated fatty acid decarboxylation for alkene production
Abstract
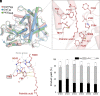
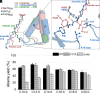
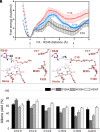
The enzymatic decarboxylation of fatty acids (FAs) represents an advance toward the development of biological routes to produce drop-in hydrocarbons. The current mechanism for the P450-catalyzed decarboxylation has been largely established from the bacterial cytochrome P450 OleTJE. Herein, we describe OleTPRN, a poly-unsaturated alkene-producing decarboxylase that outrivals the functional properties of the model enzyme and exploits a distinct molecular mechanism for substrate binding and chemoselectivity. In addition to the high conversion rates into alkenes from a broad range of saturated FAs without dependence on high salt concentrations, OleTPRN can also efficiently produce alkenes from unsaturated (oleic and linoleic) acids, the most abundant FAs found in nature. OleTPRN performs carbon-carbon cleavage by a catalytic itinerary that involves hydrogen-atom transfer by the heme-ferryl intermediate Compound I and features a hydrophobic cradle at the distal region of the substrate-binding pocket, not found in OleTJE, which is proposed to play a role in the productive binding of long-chain FAs and favors the rapid release of products from the metabolism of short-chain FAs. Moreover, it is shown that the dimeric configuration of OleTPRN is involved in the stabilization of the A-A' helical motif, a second-coordination sphere of the substrate, which contributes to the proper accommodation of the aliphatic tail in the distal and medial active-site pocket. These findings provide an alternative molecular mechanism for alkene production by P450 peroxygenases, creating new opportunities for biological production of renewable hydrocarbons.
Keywords: CYP152 peroxygenase; alkene production; decarboxylation activity; molecular mechanism; renewable hydrocarbons.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Keasling J., et al. , Microbial production of advanced biofuels. Nat. Rev. Microbiol. 19, 701–715 (2021). - PubMed
-
- Zhou Y. J., Kerkhoven E. J., Nielsen J., Barriers and opportunities in bio-based production of hydrocarbons. Nat. Energy 3, 925–935 (2018).
-
- Liao J. C., Mi L., Pontrelli S., Luo S., Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 14, 288–304 (2016). - PubMed
-
- Keasling J., et al. , Microbial production of advanced biofuels. Nat. Rev. Microbiol. 19, 701–715 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

