Therapeutic antagonism of the neurokinin 1 receptor in endosomes provides sustained pain relief
- PMID: 37216510
- PMCID: PMC10235985
- DOI: 10.1073/pnas.2220979120
Therapeutic antagonism of the neurokinin 1 receptor in endosomes provides sustained pain relief
Abstract
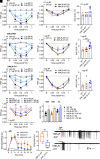
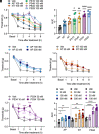
The hypothesis that sustained G protein-coupled receptor (GPCR) signaling from endosomes mediates pain is based on studies with endocytosis inhibitors and lipid-conjugated or nanoparticle-encapsulated antagonists targeted to endosomes. GPCR antagonists that reverse sustained endosomal signaling and nociception are needed. However, the criteria for rational design of such compounds are ill-defined. Moreover, the role of natural GPCR variants, which exhibit aberrant signaling and endosomal trafficking, in maintaining pain is unknown. Herein, substance P (SP) was found to evoke clathrin-mediated assembly of endosomal signaling complexes comprising neurokinin 1 receptor (NK1R), Gαq/i, and βarrestin-2. Whereas the FDA-approved NK1R antagonist aprepitant induced a transient disruption of endosomal signals, analogs of netupitant designed to penetrate membranes and persist in acidic endosomes through altered lipophilicity and pKa caused sustained inhibition of endosomal signals. When injected intrathecally to target spinal NK1R+ve neurons in knockin mice expressing human NK1R, aprepitant transiently inhibited nociceptive responses to intraplantar injection of capsaicin. Conversely, netupitant analogs had more potent, efficacious, and sustained antinociceptive effects. Mice expressing C-terminally truncated human NK1R, corresponding to a natural variant with aberrant signaling and trafficking, displayed attenuated SP-evoked excitation of spinal neurons and blunted nociceptive responses to SP. Thus, sustained antagonism of the NK1R in endosomes correlates with long-lasting antinociception, and domains within the C-terminus of the NK1R are necessary for the full pronociceptive actions of SP. The results support the hypothesis that endosomal signaling of GPCRs mediates nociception and provides insight into strategies for antagonizing GPCRs in intracellular locations for the treatment of diverse diseases.
Keywords: endocytosis; pain; receptors; signaling.
Conflict of interest statement
N.W.B. is a founding scientist of Endosome Therapeutics Inc. Research in N.W.B.’s laboratory is funded, in part, by Takeda Pharmaceuticals International.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

