Reproductive inequality in humans and other mammals
- PMID: 37216525
- PMCID: PMC10235947
- DOI: 10.1073/pnas.2220124120
Reproductive inequality in humans and other mammals
Abstract
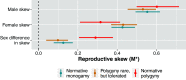
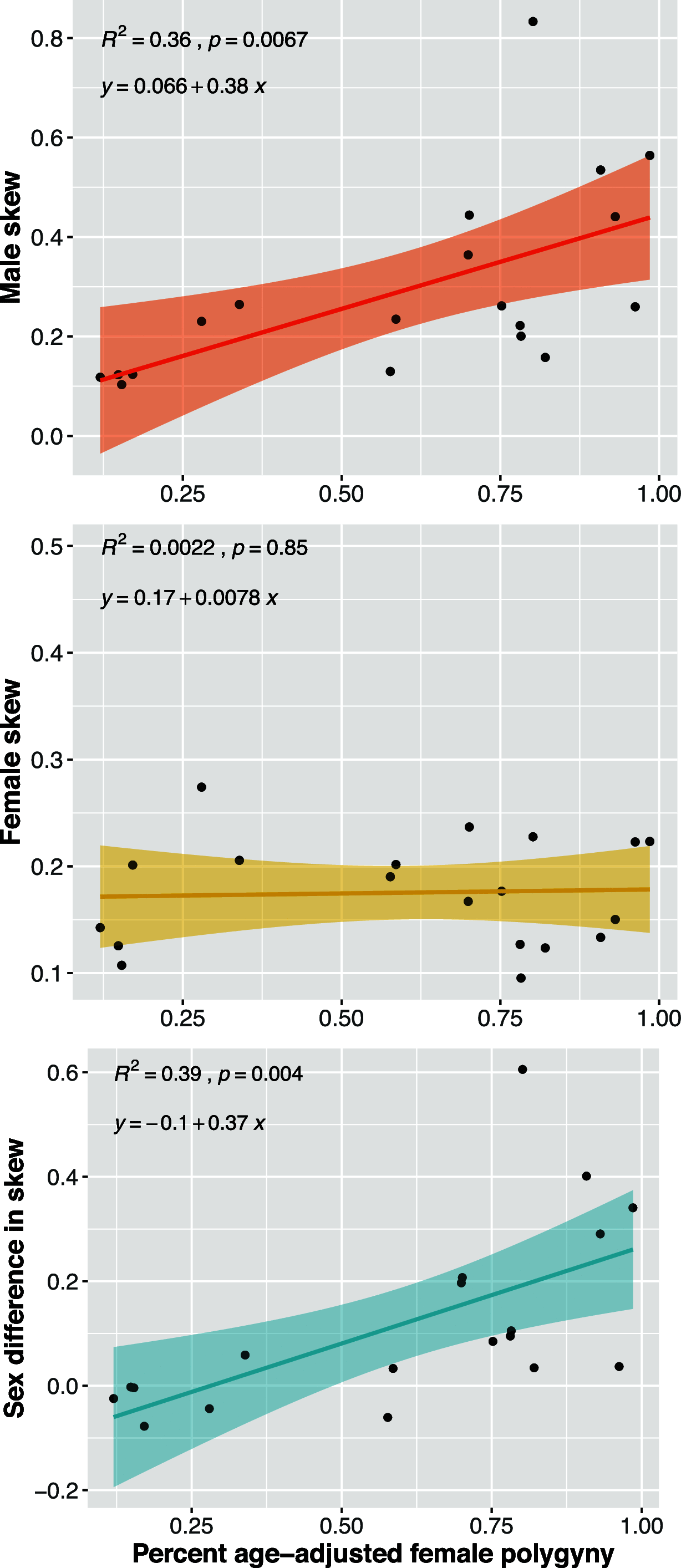
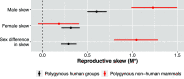
To address claims of human exceptionalism, we determine where humans fit within the greater mammalian distribution of reproductive inequality. We show that humans exhibit lower reproductive skew (i.e., inequality in the number of surviving offspring) among males and smaller sex differences in reproductive skew than most other mammals, while nevertheless falling within the mammalian range. Additionally, female reproductive skew is higher in polygynous human populations than in polygynous nonhumans mammals on average. This patterning of skew can be attributed in part to the prevalence of monogamy in humans compared to the predominance of polygyny in nonhuman mammals, to the limited degree of polygyny in the human societies that practice it, and to the importance of unequally held rival resources to women's fitness. The muted reproductive inequality observed in humans appears to be linked to several unusual characteristics of our species-including high levels of cooperation among males, high dependence on unequally held rival resources, complementarities between maternal and paternal investment, as well as social and legal institutions that enforce monogamous norms.
Keywords: egalitarian syndrome; inequality; mating systems; monogamy; reproductive skew.
Conflict of interest statement
The authors declare no competing interest.
Figures
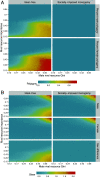
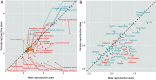
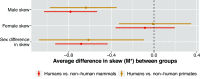
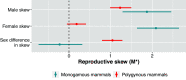

References
-
- Richerson P. J., Boyd R., Not by Genes Alone: How Culture Transformed Human Evolution (University of Chicago Press, 2008).
-
- C. P. van Schaik, J. M. Burkart, “Cooperative breeding and the evolution of our unique features” in Mind the Gap, P. M. Kappeler, J. B. Silk, Eds. (Springer, 2010), pp. 477–496.
-
- Bowles S., Gintis H., A Cooperative Species (Princeton University Press, 2011).
-
- Bramble D. M., Lieberman D. E., Endurance running and the evolution of Homo. Nature 432, 345–352 (2004). - PubMed
-
- Kaplan H., Hill K., Lancaster J., Hurtado A. M., A theory of human life history evolution. Evol. Anthropol. 9, 156–185 (2000).

