Primary cilia TRP channel regulates hippocampal excitability
- PMID: 37216541
- PMCID: PMC10235993
- DOI: 10.1073/pnas.2219686120
Primary cilia TRP channel regulates hippocampal excitability
Abstract
Polycystins (PKD2, PKD2L1, and PKD2L2) are members of the transient receptor potential family, which form ciliary ion channels. Most notably, PKD2 dysregulation in the kidney nephron cilia is associated with polycystic kidney disease, but the function of PKD2L1 in neurons is undefined. In this report, we develop animal models to track the expression and subcellular localization of PKD2L1 in the brain. We discover that PKD2L1 localizes and functions as a Ca2+ channel in the primary cilia of hippocampal neurons that apically radiate from the soma. Loss of PKD2L1 expression ablates primary ciliary maturation and attenuates neuronal high-frequency excitability, which precipitates seizure susceptibility and autism spectrum disorder-like behavior in mice. The disproportionate impairment of interneuron excitability suggests that circuit disinhibition underlies the neurophenotypic features of these mice. Our results identify PKD2L1 channels as regulators of hippocampal excitability and the neuronal primary cilia as organelle mediators of brain electrical signaling.
Keywords: TRP channels; channelopathy; ciliopathy; polycystins; primary cilia.
Conflict of interest statement
The authors declare no competing interest.
Figures
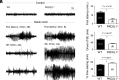

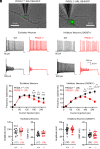
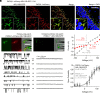
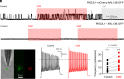
References
-
- Duncan D., Electron microscope study of the embryonic neural tube and notochord. Tex. Rep. Biol. Med. 15, 367–377 (1957). https://pubmed.ncbi.nlm.nih.gov/13495942/. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

