Hypochlorite-induced oxidation promotes aggregation and reduces toxicity of amyloid beta 1-42
- PMID: 37216700
- PMCID: PMC10209884
- DOI: 10.1016/j.redox.2023.102736
Hypochlorite-induced oxidation promotes aggregation and reduces toxicity of amyloid beta 1-42
Erratum in
-
Corrigendum to 'Hypochlorite-induced oxidation promotes aggregation and reduces toxicity of amyloid beta 1-42' [Redox Biol. 63 (2023) 102736].Redox Biol. 2023 Jun 8:102768. doi: 10.1016/j.redox.2023.102768. Online ahead of print. Redox Biol. 2023. PMID: 37301666 No abstract available.
Abstract
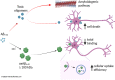
Exacerbated hypochlorite (OCl-) production is linked to neurodegenerative processes, but there is growing evidence that lower levels of hypochlorite activity are important to protein homeostasis. In this study we characterise the effects of hypochlorite on the aggregation and toxicity of amyloid beta peptide 1-42 (Aβ1-42), a major component of amyloid plaques that form in the brain in Alzheimer's disease. Our results demonstrate that treatment with hypochlorite promotes the formation of Aβ1-42 assemblies ≥100 kDa that have reduced surface exposed hydrophobicity compared to the untreated peptide. This effect is the result of the oxidation of Aβ1-42 at a single site as determined by mass spectrometry analysis. Although treatment with hypochlorite promotes the aggregation of Aβ1-42, the solubility of the peptide is enhanced and amyloid fibril formation is inhibited as assessed by filter trap assay, thioflavin T assay and transmission electron microscopy. The results of in vitro assays using SH-SY5Y neuroblastoma cells show that pre-treatment of Aβ1-42 with a sub-stoichiometric amount of hypochlorite substantially reduces its toxicity. The results of flow cytometry analysis and internalisation assays indicate that hypochlorite-induced modification of Aβ1-42 reduces its toxicity via at least two-distinct mechanism, reducing the total binding of Aβ1-42 to the surface of cells and facilitating the cell surface clearance of Aβ1-42 to lysosomes. Our data is consistent with a model in which tightly regulated production of hypochlorite in the brain is protective against Aβ-induced toxicity.
Keywords: Alzheimer's Disease; Renewable energy; inflammation; oxidative stress; post-translational modification; protein misfolding.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Johnstone M., et al. A central role for astrocytes in the inflammatory response to β-amyloid; chemokines, cytokines and reactive oxygen species are produced. J. Neuroimmunol. 1999;93(1–2):182–193. - PubMed
-
- Uddin M.S., et al. Pharmacological approaches to mitigate neuroinflammation in Alzheimer's disease. Int. Immunopharm. 2020;84 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

