Comparison of a Urine Antigen Assay and Multiple Examinations with the Formalin-Ethyl Acetate Concentration Technique for Diagnosis of Opisthorchiasis
- PMID: 37217166
- PMCID: PMC10324020
- DOI: 10.4269/ajtmh.23-0132
Comparison of a Urine Antigen Assay and Multiple Examinations with the Formalin-Ethyl Acetate Concentration Technique for Diagnosis of Opisthorchiasis
Abstract
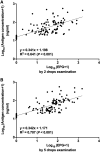
Detection of worm antigen in urine is a sensitive diagnostic method for opisthorchiasis, particularly for light-intensity infections; however, the presence of eggs in feces is essential for validating results from the antigen assay. To address the issue of low sensitivity of fecal examination, we modified the protocol for the formalin-ethyl acetate concentration technique (FECT) and compared it against urine antigen measurements for detection of the parasite Opisthorchis viverrini. First, we optimized the FECT protocol by increasing the number of drops for examinations from the standard two drops to a maximum of eight. We were able to detect additional cases after examination of ≥ 3 drops, and the prevalence of O. viverrini saturated after examination of ≥ 5 drops. We then compared the optimized FECT protocol (examining five drops of suspension) against urine antigen detection for the diagnosis of opisthorchiasis in field-collected samples. The optimized FECT protocol detected O. viverrini eggs in 25 of 82 individuals (30.5%) who had positive urine antigen tests but were fecal egg negative by the standard FECT protocol. The optimized protocol also retrieved O. viverrini eggs in 2 of 80 antigen-negative cases (2.5%). In comparison with the composite reference standard (combined FECT and urine antigen detection), the diagnostic sensitivity of examining two and five drops of FECT and the urine assay was 58.2, 67, and 98.8%, respectively. Our results show that multiple examinations of fecal sediment increase the diagnostic sensitivity of FECT and thus provide further support for the reliability and utility of the antigen assay for diagnosis and screening of opisthorchiasis.
Figures

References
-
- WHO , 2021. Ending the Neglect to Attain the Sustainable Development Goals: A Sustainability Framework for Action against Neglected Tropical Diseases 2021–2030. Geneva, Switzerland: World Health Organization.
-
- International Agency for Research on Cancer (IARC) , 2012. A review of human carcinogens: biological agents. IARC Monogr Eval Carcinog Risks Hum 100: 341–370.
-
- Bouvard V. et al. , 2009. A review of human carcinogens – part B: biological agents. Lancet Oncol 10: 321–322. - PubMed
-
- Crellen T, Sithithaworn P, Pitaksakulrat O, Khuntikeo N, Medley GF, Hollingsworth TD, 2021. Towards evidence-based control of Opisthorchis viverrini . Trends Parasitol 37: 370–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

