Long-term follow-up of anti-PD-1 naïve patients with metastatic melanoma treated with IDO/PD-L1 targeting peptide vaccine and nivolumab
- PMID: 37217243
- PMCID: PMC10230976
- DOI: 10.1136/jitc-2023-006755
Long-term follow-up of anti-PD-1 naïve patients with metastatic melanoma treated with IDO/PD-L1 targeting peptide vaccine and nivolumab
Abstract
Background: We have previously published initial efficacy of the indoleamine 2,3-dioxygenase (IDO)/anti-programmed death ligand 1 (PD-L1) vaccine in combination with nivolumab in 30 anti-PD-1 therapy naïve patients with metastatic melanoma (cohort A). We now report long-term follow-up of patients in cohort A. Further, we report results from cohort B, where the peptide vaccine was added to anti-PD-1 therapy for patients with progressive disease during anti-PD-1 treatment.
Methods: All patients were treated with a therapeutic peptide vaccine in Montanide targeting IDO and PD-L1 combined with nivolumab (NCT03047928). A long-term follow-up of safety, response rates, and survival rates were performed in cohort A including patient subgroup analyses. Safety and clinical responses were analyzed for cohort B.
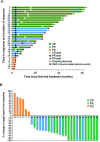
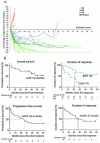
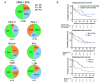
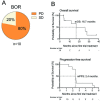
Results: Cohort A: At data cut-off, January 5, 2023, the overall response rate (ORR) was 80%, and 50% of the 30 patients obtained a complete response (CR). The median progression-free survival (mPFS) was 25.5 months (95% CI 8.8 to 39), and median overall survival (mOS) was not reached (NR) (95% CI 36.4 to NR). The minimum follow-up time was 29.8 months, and the median follow-up was 45.3 months (IQR 34.8-59.2). A subgroup evaluation further revealed that cohort A patients with unfavorable baseline characteristics, including either PD-L1 negative tumors (n=13), elevated lactate dehydrogenase (LDH) levels (n=11), or M1c (n=17) obtained both favorable response rates and durable responses. The ORR was 61.5%, 79%, and 88% for patients with PD-L1- tumors, elevated LDH, and M1c, respectively. The mPFS was 7.1 months for patients with PD-L1- tumors, 30.9 months for patients with elevated LDH, and 27.9 months for M1c patients. Cohort B: At data cut-off, the best overall response was stable disease for 2 of the 10 evaluable patients. The mPFS was 2.4 months (95% CI 1.38 to 2.52), and the mOS was 16.7 months (95% CI 4.13 to NR).
Conclusion: This long-term follow-up confirms the promising and durable responses in cohort A. Subgroup analyses of patients with unfavorable baseline characteristics revealed that high response rates and survival rates were also found in patients with either PD-L1 negative tumors, elevated LDH levels, or M1c. No meaningful clinical effect was demonstrated in cohort B patients.
Trial registration number: NCT03047928.
Keywords: immunotherapy; melanoma; nivolumab; vaccination.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MHA has patent applications allocated to the biotech company IO Biotech ApS concerning IDO and PD-L1 peptide therapy. MHA is a shareholder, founder, and advisor for the company IO Biotech. EE is employed at IO Biotech. IMS has either lectured for or had relationships regarding advisory board with Novo Nordisk, MSD, Novartis, Pierre Fabre Sanofi Aventis, BMS, IO Biotech, and TILT Biotherapeutics. IMS received research grants from BMS, IO Biotech, Adaptimmune, Lytix biopharma, and TILT Biotherapeutics. IMS is the shareholder and cofounder of the biotech company IO Biotech ApS. CLL and JWK do not have conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
