Variation in histone configurations correlates with gene expression across nine inbred strains of mice
- PMID: 37217254
- PMCID: PMC10519406
- DOI: 10.1101/gr.277467.122
Variation in histone configurations correlates with gene expression across nine inbred strains of mice
Abstract
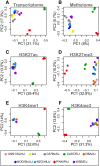
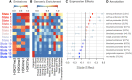
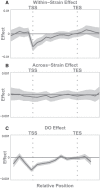
The Diversity Outbred (DO) mice and their inbred founders are widely used models of human disease. However, although the genetic diversity of these mice has been well documented, their epigenetic diversity has not. Epigenetic modifications, such as histone modifications and DNA methylation, are important regulators of gene expression and, as such, are a critical mechanistic link between genotype and phenotype. Therefore, creating a map of epigenetic modifications in the DO mice and their founders is an important step toward understanding mechanisms of gene regulation and the link to disease in this widely used resource. To this end, we performed a strain survey of epigenetic modifications in hepatocytes of the DO founders. We surveyed four histone modifications (H3K4me1, H3K4me3, H3K27me3, and H3K27ac), as well as DNA methylation. We used ChromHMM to identify 14 chromatin states, each of which represents a distinct combination of the four histone modifications. We found that the epigenetic landscape is highly variable across the DO founders and is associated with variation in gene expression across strains. We found that epigenetic state imputed into a population of DO mice recapitulated the association with gene expression seen in the founders, suggesting that both histone modifications and DNA methylation are highly heritable mechanisms of gene expression regulation. We illustrate how DO gene expression can be aligned with inbred epigenetic states to identify putative cis-regulatory regions. Finally, we provide a data resource that documents strain-specific variation in the chromatin state and DNA methylation in hepatocytes across nine widely used strains of laboratory mice.
© 2023 Tyler et al.; Published by Cold Spring Harbor Laboratory Press.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
