Advances in Immunocompetent Mouse and Rat Models
- PMID: 37217281
- PMCID: PMC10810718
- DOI: 10.1101/cshperspect.a041328
Advances in Immunocompetent Mouse and Rat Models
Abstract
Rodent models of breast cancer have played critical roles in our understanding of breast cancer development and progression as well as preclinical testing of cancer prevention and therapeutics. In this article, we first review the values and challenges of conventional genetically engineered mouse (GEM) models and newer iterations of these models, especially those with inducible or conditional regulation of oncogenes and tumor suppressors. Then, we discuss nongermline (somatic) GEM models of breast cancer with temporospatial control, made possible by intraductal injection of viral vectors to deliver oncogenes or to manipulate the genome of mammary epithelial cells. Next, we introduce the latest development in precision editing of endogenous genes using in vivo CRISPR-Cas9 technology. We conclude with the recent development in generating somatic rat models for modeling estrogen receptor-positive breast cancer, something that has been difficult to accomplish in mice.
Copyright © 2024 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
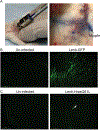
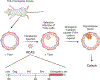



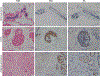
Similar articles
-
Efficient cancer modeling through CRISPR-Cas9/HDR-based somatic precision gene editing in mice.Sci Adv. 2023 May 12;9(19):eade0059. doi: 10.1126/sciadv.ade0059. Epub 2023 May 12. Sci Adv. 2023. PMID: 37172086 Free PMC article.
-
Rat Models of Breast Cancer.Adv Exp Med Biol. 2025;1464:123-148. doi: 10.1007/978-3-031-70875-6_8. Adv Exp Med Biol. 2025. PMID: 39821024 Review.
-
Intraductal Injection of Lentivirus Vectors for Stably Introducing Genes into Rat Mammary Epithelial Cells in Vivo.J Mammary Gland Biol Neoplasia. 2020 Dec;25(4):389-396. doi: 10.1007/s10911-020-09469-w. Epub 2020 Nov 9. J Mammary Gland Biol Neoplasia. 2020. PMID: 33165800 Free PMC article.
-
In situ CRISPR-Cas9 base editing for the development of genetically engineered mouse models of breast cancer.EMBO J. 2020 Mar 2;39(5):e102169. doi: 10.15252/embj.2019102169. Epub 2020 Jan 13. EMBO J. 2020. PMID: 31930530 Free PMC article.
-
CRISPR-Cas9 therapies in experimental mouse models of cancer.Future Oncol. 2018 Aug;14(20):2083-2095. doi: 10.2217/fon-2018-0028. Epub 2018 Jul 20. Future Oncol. 2018. PMID: 30027767 Review.
Cited by
-
SREBP1-Dependent Metabolism as a Potential Target for Breast Cancer Risk Reduction.Cancers (Basel). 2025 May 14;17(10):1664. doi: 10.3390/cancers17101664. Cancers (Basel). 2025. PMID: 40427160 Free PMC article. Review.
-
Efficient cancer modeling through CRISPR-Cas9/HDR-based somatic precision gene editing in mice.Sci Adv. 2023 May 12;9(19):eade0059. doi: 10.1126/sciadv.ade0059. Epub 2023 May 12. Sci Adv. 2023. PMID: 37172086 Free PMC article.
-
The Pivotal Role of Preclinical Animal Models in Anti-Cancer Drug Discovery and Personalized Cancer Therapy Strategies.Pharmaceuticals (Basel). 2024 Aug 9;17(8):1048. doi: 10.3390/ph17081048. Pharmaceuticals (Basel). 2024. PMID: 39204153 Free PMC article. Review.
-
Severe degranulation of mesenteric mast cells in an experimental rat mammary tumor model.Turk J Med Sci. 2024 Sep 10;54(6):1381-1388. doi: 10.55730/1300-0144.5921. eCollection 2024. Turk J Med Sci. 2024. PMID: 39734347 Free PMC article.
-
Rat Models of Breast Cancer.Adv Exp Med Biol. 2025;1464:123-148. doi: 10.1007/978-3-031-70875-6_8. Adv Exp Med Biol. 2025. PMID: 39821024 Review.
References
-
- Ando S, Malivindi R, Catalano S, Rizza P, Barone I, Panza S, Rovito D, Emprou C, Bornert JM, Laverny G et al. 2017. Conditional expression of Ki-Ras(G12V) in the mammary epithelium of transgenic mice induces estrogen receptor alpha (ERalpha)-positive adenocarcinoma. Oncogene 36: 6420–6431. - PubMed
-
- Andres AC, Schonenberger CA, Groner B, Hennighausen L, LeMeur M, Gerlinger P. 1987. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America 84: 1299–1303. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
