A non-randomized risk-adjusted comparison of lenalidomide + R-CHOP versus R-CHOP for MYC-rearranged DLBCL patients
- PMID: 37217463
- PMCID: PMC10203347
- DOI: 10.1038/s41408-023-00854-2
A non-randomized risk-adjusted comparison of lenalidomide + R-CHOP versus R-CHOP for MYC-rearranged DLBCL patients
Abstract
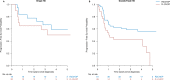
Patients with MYC rearranged (MYC-R) diffuse large B-cell lymphoma (DLBCL) have a poor prognosis. Previously, we demonstrated in a single-arm phase II trial (HOVON-130) that addition of lenalidomide to R-CHOP (R2CHOP) is well-tolerated and yields similar complete metabolic remission rates as more intensive chemotherapy regimens in literature. In parallel with this single-arm interventional trial, a prospective observational screening cohort (HOVON-900) was open in which we identified all newly diagnosed MYC-R DLBCL patients in the Netherlands. Eligible patients from the observational cohort that were not included in the interventional trial served as control group in the present risk-adjusted comparison. R2CHOP treated patients from the interventional trial (n = 77) were younger than patients in the R-CHOP control cohort (n = 56) (median age 63 versus 70 years, p = 0.018) and they were more likely to have a lower WHO performance score (p = 0.013). We adjusted for differences at baseline using 1:1 matching, multivariable analysis, and weighting using the propensity score to reduce treatment-selection bias. These analyses consistently showed improved outcome after R2CHOP with HRs of 0.53, 0.51, and 0.59, respectively, for OS, and 0.53, 0.59, and 0.60 for PFS. Thus, this non-randomized risk-adjusted comparison supports R2CHOP as an additional treatment option for MYC-R DLBCL patients.
© 2023. The Author(s).
Conflict of interest statement
AVDJ, EVW, AGD, MN, AHZ, PMB, MSV, EGGMR, RM, JSPV, YS, EDJ, YM, RB, HK, and DDJ declare no competing financial interests. MJK received honoraria from Kite, Novartis, and Miltenyi Biotech, Roche, and Bristol Myers Squibb/Celgene; consultancy or advisory role for Kite, Roche, Bristol Myers Squibb/Celgene, Novartis, and Miltenyi Biotech; research funding from Kite, Roche, Takeda, and Celgene (all to institution); and travel support from Kite, Roche, Novartis, and Miltenyi Biotech. MEDC received research funding from BMS/Celgene, Gilead and GenMAb. Advisory role for AbbVie and Novartis.
Figures




References
-
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94. 10.1056/NEJM199309303291402. - PubMed
-
- Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61. doi: 10.1182/blood-2006-08-038257. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

