Anion redox as a means to derive layered manganese oxychalcogenides with exotic intergrowth structures
- PMID: 37217479
- PMCID: PMC10202913
- DOI: 10.1038/s41467-023-38489-3
Anion redox as a means to derive layered manganese oxychalcogenides with exotic intergrowth structures
Abstract
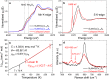
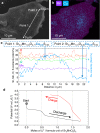
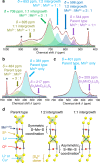
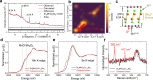
Topochemistry enables step-by-step conversions of solid-state materials often leading to metastable structures that retain initial structural motifs. Recent advances in this field revealed many examples where relatively bulky anionic constituents were actively involved in redox reactions during (de)intercalation processes. Such reactions are often accompanied by anion-anion bond formation, which heralds possibilities to design novel structure types disparate from known precursors, in a controlled manner. Here we present the multistep conversion of layered oxychalcogenides Sr2MnO2Cu1.5Ch2 (Ch = S, Se) into Cu-deintercalated phases where antifluorite type [Cu1.5Ch2]2.5- slabs collapsed into two-dimensional arrays of chalcogen dimers. The collapse of the chalcogenide layers on deintercalation led to various stacking types of Sr2MnO2Ch2 slabs, which formed polychalcogenide structures unattainable by conventional high-temperature syntheses. Anion-redox topochemistry is demonstrated to be of interest not only for electrochemical applications but also as a means to design complex layered architectures.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that none of them have any competing financial, personal or professional relationships with individuals or institutions that could be perceived to directly undermine the objectivity, integrity, and value of the work in this article, or could be seen as having an influence on the judgments and actions of the authors with regard to objective data presentation, analysis, and interpretation.
Figures



References
-
- Rouxel J. Anion–cation redox competition and the formation of new compounds in highly covalent systems. Chem. Eur. J. 1996;2:1053–1059. doi: 10.1002/chem.19960020904. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

