Nonthermal acceleration of protein hydration by sub-terahertz irradiation
- PMID: 37217486
- PMCID: PMC10203368
- DOI: 10.1038/s41467-023-38462-0
Nonthermal acceleration of protein hydration by sub-terahertz irradiation
Abstract
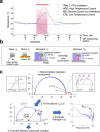
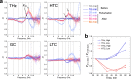
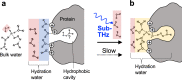
The collective intermolecular dynamics of protein and water molecules, which overlap in the sub-terahertz (THz) frequency region, are relevant for expressing protein functions but remain largely unknown. This study used dielectric relaxation (DR) measurements to investigate how externally applied sub-THz electromagnetic fields perturb the rapid collective dynamics and influence the considerably slower chemical processes in protein-water systems. We analyzed an aqueous lysozyme solution, whose hydration is not thermally equilibrated. By detecting time-lapse differences in microwave DR, we demonstrated that sub-THz irradiation gradually decreases the dielectric permittivity of the lysozyme solution by reducing the orientational polarization of water molecules. Comprehensive analysis combining THz and nuclear magnetic resonance spectroscopies suggested that the gradual decrease in the dielectric permittivity is not induced by heating but is due to a slow shift toward the hydrophobic hydration structure in lysozyme. Our findings can be used to investigate hydration-mediated protein functions based on sub-THz irradiation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

